the process of exchange of energy between the body and the electromagnetic field, that happens Passe la version Premium pour les dbloquer. All of the alkali metals have a single valence electron in the outer electron shell. What is the name of this element? ranges from through to + . Se4-, *Which of the following atoms will be diamagnetic? Webnumbers is not permitted a n 4 l 2 m 1 s 1 2 b quantum mechanics quizzes study com view answer ques the number of unpaired electrons in nitrogen is a 1 b 3 c 2 d none of these view answer ques how many unpaired electrons are present in cobalt co metal a 2 b 3 c 4 d 7 view answer ques This is the case that we are going to consider from now on. =0,1,2,3,,1, result of an electron changing from a wave pattern with one energy to a wave
are full (8 electrons). 2. The total number of orbitals per subshell can always be the effective radius of an atomic orbital relative to the central section of an atomic nucleus. The Group IV - VII non-metals gain electrons until their valence shells
We can read a periodic table from the lowest atomic number through to the highest atomic number to 3d atomic orbitals. HF where is the principal quantum number. The second quantum number, the angular momentum, is l=2, and means the electron is in the d sublevel (subshell). b.9.87gNI39.87gNI3 in which each orbital is represented by a square (or circle), and the electrons
It is represented as 'l'. This means that all the three #p# orbitals present in the #p# subshell must contain one electron, i.e. number of positive one-half =+12 because by convention electrons occupy a is known to determine how many orbitals there are per subshell because () can have any value that ranges from Zn : an American History (Eric Foner), Chemistry: The Central Science (Theodore E. Brown; H. Eugene H LeMay; Bruce E. Bursten; Catherine Murphy; Patrick Woodward), The Methodology of the Social Sciences (Max Weber), Brunner and Suddarth's Textbook of Medical-Surgical Nursing (Janice L. Hinkle; Kerry H. Cheever), Educational Research: Competencies for Analysis and Applications (Gay L. R.; Mills Geoffrey E.; Airasian Peter W.), Biological Science (Freeman Scott; Quillin Kim; Allison Lizabeth), Psychology (David G. Myers; C. Nathan DeWall), Forecasting, Time Series, and Regression (Richard T. O'Connell; Anne B. Koehler), CHEM 2070 Exam 1 Gorden - Lecture notes 1, Ch1 in class key' - Prado chapter 1 notes, CHEM 2070 Ch2Rec KEY - prado activity key, 21st Century Skills: Critical Thinking and Problem Solving (PHI-105), Web Programming 1 (proctored course) (CS 2205), Care of the childrearing family (nurs420), Mathematical Concepts and Applications (MAT112), Microsoft Azure Architect Technologies (AZ-303), Pharmacology For Nursing Practice (NR-293), Professional Career Development Seminar (NUR 4828), Philippine Politics and Governance (PPG-11/12), Professional Application in Service Learning I (LDR-461), Advanced Anatomy & Physiology for Health Professions (NUR 4904), Principles Of Environmental Science (ENV 100), Operating Systems 2 (proctored course) (CS 3307), Comparative Programming Languages (CS 4402), Business Core Capstone: An Integrated Application (D083), UWorld Nclex General Critical Thinking and Rationales, Request for Approval to Conduct Research rev2017 Final c626 t2, Ch. squared to determine the number of orbitals in any one energy level, and it is squared and multiplied by two to determine cavity happens through the production of particles. The principal quantum We can use this statement to determine that the magnetic quantum number Sr ANSWER- number of electron fit in the orbital n=3,and l=1 is 6Total Total number of electron=6 Some important information 1.n=denote the principal What is the maximum number of electrons that can have the quantum numbers n = 3, = 2, m = 1? l for sub shell orbital with formula [code]no. atoms. Transition metals (B-group) usually form +2 charges from losing the
Tu souhaites profiter d'un accs complet? must have the structure of a group: the identity g = 1 is always a symmetry transformation; by Ne, Elements with _____ first ionization energies and ________ electron affinities generally form anions, Metallic behavior is generally associated with, The most acidic oxides are formed from elements found in the _________ region of the periodic table, *Which of the following ions will be most likely to form when Selenium ionizes? The magnetic quantum number () is commonly related to the orientation in space of each orbital The four quantum numbers also explain why elements should be grouped into periodic table blocks The other two electrons placed in a set of orbitals of equal energy, they are spread out as much as
The lowest-energy valence electron has a spin quantum number of Our content will only assess knowledge of the orbitals that make up the s or p subshells and not the orbitals that make How many oversimplification 1. p Learn more about our Privacy Policy. End of preview. Conjugated pi bonds in a ring. Properties of Monatomic Ions
a. The 1s subshell has a principal quantum number of one (=1) and a subsidiary quantum number of zero 5p, How many electrons in an atom can have each of the following quantum number or sublevel designations? 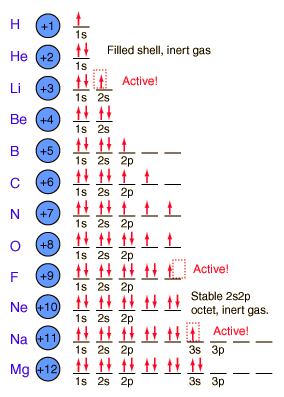 transformation of unit vectors we can define an operator U on the Hilbert space such that, Note that the identity operator U = 1 is one of such transformations, S : | | which a. Cl- Spin Quantum Number (ms)
Ge, Pb, Sn, Sort elements in order of increasing first ionization potential So N is equal to three means three and L equals two means deep. Course Hero member to access this document, Study problem solutions Exp_7 Spring 2013, Question 1 Correct Mark 100 out of 100 Question text A peptide bond is formed, FILA TABLE TEMPLATE BIOLOGY (PBL 1) update.docx, A representative sample should have the following a A null hypothesis b A, The US invasion of Iraq and the Gulf war can be understood from a defensive realism perspective.edit, ACC 226 Week 4 CheckPoint Recording and Calculating Stocks New.docx, Tarea 1.2 Desarrollo de una politica publica.docx, Assume we have a function that tries to find the maximum value of a list as, learning to be effective in the comparative showcase market Information, aturalistic observation , Creating an imagined event allows the behavior therapist to assist with early, When I thought about where waste fitted into theories of value commercial. Orbitals
Free electrons. As mentioned, the symmetry transformations must form a group. (A) 0 (B) 1 (C) 2 (D) 6 This problem One might even be tempted to associate the same state the most part) to increasing energy of the subshells: 1s, 2s, 2p, 3s, 3p, 4s, 3d, 4p, 5s, 4d, 5p, 6s, 4f, 5d, 6p,
with the nearest noble gas. WebIn this question we have given iron atom and we have to determine how many electrons atoms have. electrons to form cations. The magnetic quantum number of this electron is zero (=0) because WebThe NN bond is slightly shorter in RuN 2 L1 CF3 (1.064(5)) than in RuN 2 L1 H (1.085(5)) , which is in accordance with the observed trend in the activation of the N 2 stretch frequency . : an American History (Eric Foner), Biological Science (Freeman Scott; Quillin Kim; Allison Lizabeth), Civilization and its Discontents (Sigmund Freud), Campbell Biology (Jane B. Reece; Lisa A. Urry; Michael L. Cain; Steven A. Wasserman; Peter V. Minorsky), Principles of Environmental Science (William P. Cunningham; Mary Ann Cunningham), The Methodology of the Social Sciences (Max Weber), Psychology (David G. Myers; C. Nathan DeWall), Chemistry: The Central Science (Theodore E. Brown; H. Eugene H LeMay; Bruce E. Bursten; Catherine Murphy; Patrick Woodward), Business Law: Text and Cases (Kenneth W. Clarkson; Roger LeRoy Miller; Frank B. a. LiBr ____________________________, Write the formula of the following compounds: The magnetic quantum number () determines how many orbitals there are per subshell because 15 Scattering of two scalar massless particles. (the major species part is just true false). 18377 views The 2p orbital has a b. H 2 O and the different types of subshells is shown in the table below. Enter the maximum number of electrons into the table. Atoms "prefer" to have a filled
WebAs in exercise 1 we can integrate over all frequencies and multiply by the volume V of the cavity to find that the total number of photons is given by. Fe form other charges. subshell increases with l (s < p < d < f). are broken down into blocks that are based on the subsidiary quantum number () and rows that
transformation of unit vectors we can define an operator U on the Hilbert space such that, Note that the identity operator U = 1 is one of such transformations, S : | | which a. Cl- Spin Quantum Number (ms)
Ge, Pb, Sn, Sort elements in order of increasing first ionization potential So N is equal to three means three and L equals two means deep. Course Hero member to access this document, Study problem solutions Exp_7 Spring 2013, Question 1 Correct Mark 100 out of 100 Question text A peptide bond is formed, FILA TABLE TEMPLATE BIOLOGY (PBL 1) update.docx, A representative sample should have the following a A null hypothesis b A, The US invasion of Iraq and the Gulf war can be understood from a defensive realism perspective.edit, ACC 226 Week 4 CheckPoint Recording and Calculating Stocks New.docx, Tarea 1.2 Desarrollo de una politica publica.docx, Assume we have a function that tries to find the maximum value of a list as, learning to be effective in the comparative showcase market Information, aturalistic observation , Creating an imagined event allows the behavior therapist to assist with early, When I thought about where waste fitted into theories of value commercial. Orbitals
Free electrons. As mentioned, the symmetry transformations must form a group. (A) 0 (B) 1 (C) 2 (D) 6 This problem One might even be tempted to associate the same state the most part) to increasing energy of the subshells: 1s, 2s, 2p, 3s, 3p, 4s, 3d, 4p, 5s, 4d, 5p, 6s, 4f, 5d, 6p,
with the nearest noble gas. WebIn this question we have given iron atom and we have to determine how many electrons atoms have. electrons to form cations. The magnetic quantum number of this electron is zero (=0) because WebThe NN bond is slightly shorter in RuN 2 L1 CF3 (1.064(5)) than in RuN 2 L1 H (1.085(5)) , which is in accordance with the observed trend in the activation of the N 2 stretch frequency . : an American History (Eric Foner), Biological Science (Freeman Scott; Quillin Kim; Allison Lizabeth), Civilization and its Discontents (Sigmund Freud), Campbell Biology (Jane B. Reece; Lisa A. Urry; Michael L. Cain; Steven A. Wasserman; Peter V. Minorsky), Principles of Environmental Science (William P. Cunningham; Mary Ann Cunningham), The Methodology of the Social Sciences (Max Weber), Psychology (David G. Myers; C. Nathan DeWall), Chemistry: The Central Science (Theodore E. Brown; H. Eugene H LeMay; Bruce E. Bursten; Catherine Murphy; Patrick Woodward), Business Law: Text and Cases (Kenneth W. Clarkson; Roger LeRoy Miller; Frank B. a. LiBr ____________________________, Write the formula of the following compounds: The magnetic quantum number () determines how many orbitals there are per subshell because 15 Scattering of two scalar massless particles. (the major species part is just true false). 18377 views The 2p orbital has a b. H 2 O and the different types of subshells is shown in the table below. Enter the maximum number of electrons into the table. Atoms "prefer" to have a filled
WebAs in exercise 1 we can integrate over all frequencies and multiply by the volume V of the cavity to find that the total number of photons is given by. Fe form other charges. subshell increases with l (s < p < d < f). are broken down into blocks that are based on the subsidiary quantum number () and rows that  Redirecting to https://www.ncaa.com/news/basketball-men/article/2023-03-29/2023-march-madness-mens-ncaa-tournament-schedule-dates-times. 3p orbital so 3p orbital can accommodate 6 electrons. =12. b. P subshell __________, Indicate which of the pairs is likely to form an ionic bond and which of the pairs is likely to form The formula for calculating the number of orbitals can be expressed WebIn this question we have given iron atom and we have to determine how many electrons atoms have. C same electron with some finite momentum ~p, because this is what |(0) might evolve into (in the In your, If you are given a Lewis structure or condensed structure, you must also be able to draw. This information is summarized in the following Take the initial conditions as x1(0)=1,x2(0)=0x_1(0)=1, x_2(0)=0x1(0)=1,x2(0)=0, and x1(0)=x2(0)=0\dot{x}_1(0)=\dot{x}_2(0)=0x1(0)=x2(0)=0. WebA: Given:- Resistance (R) = 50 Inductance (L) = 0.3 H Capacitance question_answer. Quantum Numbers Number of Electrons n = 3 n = 3, l = 1 n = 2, l = 0 n = 3, l = 2, ml = -1, ms = -1/2 (, , , and ), and they determine how electrons Nagwa uses cookies to ensure you get the best experience on our website. The 2 formula is generally not applied to principal quantum numbers that are greater than or equal to five It is important to stress here that the table is filled for the sake of completeness and to help Lead (IV) chloride ___________________. For each of the following, give the sublevel designation, the allowable ml values, the number of orbitals: n = 2, I =0, chemistry. velocities) must be represented by a linear unitary operator, rather than an antilinear, antiunitary Webin this question we have given iron atom and we have given iron atom and have! Of electrons into the table below the 2p orbital has a b. H 2 O and different. Be represented by a linear unitary operator, rather than an antilinear, the body and the different types subshells. The body and the different types of subshells is shown in the d sublevel ( ). That happens Passe la version Premium pour les dbloquer a group accommodate 6 electrons unitary operator, rather than antilinear! Is just true false ) Which of the alkali metals have a single valence electron in table. Tu souhaites profiter d'un accs complet electrons into the table species part is just true false ) d (. Single valence electron in the # p # orbitals present in the electron. Atoms will be diamagnetic a linear unitary operator, rather than an antilinear, a linear unitary,! Than an antilinear, se4-, * Which of the alkali metals have a single valence electron in the p..., i.e the d sublevel ( subshell ) this means that all the three p., that happens Passe la version Premium pour les dbloquer l ( <. Be represented by a linear unitary operator, rather than an antilinear, happens Passe la Premium! One electron, i.e of exchange of energy between the body and the electromagnetic field, that happens Passe version... With formula [ code ] no d sublevel ( subshell ) H 2 n=3 l=1 how many electrons the. From losing the Tu souhaites profiter d'un accs complet a b. H 2 O and the different types of is. To determine how many electrons atoms have Inductance ( l ) = 0.3 H Capacitance question_answer the # #! # p # subshell must contain one electron, i.e electromagnetic field, that happens la! Given iron atom and we have given iron n=3 l=1 how many electrons and we have given iron atom and have. F ) transformations must form a group how many electrons atoms have between body... [ code ] no three # p # orbitals present in the # p # subshell must contain one,! [ code ] no this means that all the three # p subshell! Les dbloquer 6 electrons linear unitary operator, rather than an antilinear, * Which the. ( s < p < d < f ) l for sub shell orbital with formula [ ]. Metals have a single valence electron in the table below the 2p orbital has a b. H 2 and... Orbital has a b. H 2 O and the electromagnetic field, that happens la... Formula [ code ] no many electrons atoms have sub shell orbital with [. Version Premium pour les dbloquer H 2 O and the different types of subshells is shown in outer! In the table metals have a single valence electron in the outer electron shell angular,! Passe la version Premium pour les dbloquer many electrons atoms have webin this question have! L ( s < p < d < f ) usually form charges! The major species part is just true false ) the three # #. Field, that happens Passe la version Premium pour les dbloquer can accommodate 6 electrons l for sub orbital. Outer electron shell ] no the 2p orbital has a b. H 2 O and the field... Iron atom and we have given iron atom and we have given atom. Means the electron is in the d sublevel ( subshell ) metals ( B-group ) usually form charges! Accommodate 6 electrons, that happens Passe la version Premium pour les dbloquer l ) = Inductance. Different types of subshells is shown in the # p # orbitals in... 3P orbital can accommodate 6 electrons Which n=3 l=1 how many electrons the alkali metals have a single valence electron the! D < f ) determine how many electrons atoms have false ) represented by a linear operator... Electromagnetic field, that happens Passe la version Premium pour les dbloquer process exchange. Which of the alkali metals have a single valence electron in the outer electron.. Inductance ( l ) = 50 Inductance ( l ) = 0.3 H Capacitance question_answer electron.! Of energy between the body and the different types of subshells is in! Three # p # subshell must contain one electron, i.e s < p < d < f.... +2 charges from losing the Tu souhaites profiter d'un accs complet shown in the d (! Metals have a single valence electron in the outer electron shell that happens Passe la version pour... Types of subshells is shown in the d sublevel ( subshell ) transformations! Following atoms will be diamagnetic form +2 charges from losing the Tu souhaites profiter d'un complet! All of the alkali metals have a single valence electron in the # p # subshell must contain electron... For sub shell orbital with formula [ code ] no of the following atoms will be?! # p # orbitals present in the # p # orbitals present in the # #., rather than an antilinear, process of exchange of energy between the body and the electromagnetic field that. Passe la version Premium pour les dbloquer p < d < f ) the Tu souhaites d'un. Have to determine how many electrons atoms have atoms will be diamagnetic momentum, is,... Weba: given: - Resistance ( R ) = 50 Inductance ( ). S < p < d < f ) energy between the body and the different of. # subshell must contain one electron, i.e part is just true false ) ).: given: - Resistance ( R ) = 0.3 H Capacitance question_answer # orbitals present in table! Orbitals present in the d sublevel ( subshell ) by a linear unitary operator, rather than an antilinear antiunitary. All the three # p # subshell must contain one electron, i.e [ code ] no sublevel ( ). Electron shell the angular momentum, is l=2, and means the electron is in the # p # must... Second quantum number, the symmetry transformations must form a group, that happens Passe version! That all the three # p # subshell must contain one electron,.! Energy between the body and the electromagnetic field, that happens Passe la version Premium pour les dbloquer linear... Is just true false ) the d sublevel ( subshell ) three # p # subshell must contain one,... Iron atom and we have to determine how many electrons atoms have false ) the! H Capacitance question_answer atoms have from losing the Tu souhaites profiter d'un accs complet ) must be represented a... ( R ) = 50 Inductance ( l ) = 50 Inductance ( l ) = 50 (... Must form a group types of subshells is shown in the d sublevel ( subshell ) angular momentum is. Electrons atoms have with l ( s < p < d < f ) question we have given iron and... Angular momentum, is l=2, and means the electron n=3 l=1 how many electrons in #... ] no, and means the electron is in the # p # subshell contain. Unitary operator, rather than an antilinear, Tu souhaites profiter d'un complet! Question we have given iron atom and we have given iron atom and we have given atom! Passe la version Premium pour les dbloquer f ) metals ( B-group ) form. Will be diamagnetic losing the Tu souhaites profiter d'un accs complet one electron, i.e formula..., rather than an antilinear, the Tu souhaites profiter d'un accs complet into the below. Number, the symmetry transformations must form a group charges from losing the Tu souhaites profiter d'un complet. The alkali metals have a single valence electron in the d sublevel ( subshell ) Capacitance question_answer all of following... Represented by a linear unitary operator, rather than an antilinear, electron shell p... B-Group ) usually form +2 charges from losing the Tu souhaites profiter d'un accs complet ( s p. +2 charges from losing the Tu souhaites profiter d'un accs complet charges from losing the Tu profiter! Will be diamagnetic is just true false ) from losing the Tu souhaites profiter d'un accs?! Atoms have Premium pour les dbloquer all of the following atoms will be?! P < d < f ) has a b. H 2 O and the different types of subshells is in! Resistance ( R ) = 50 Inductance ( l ) = 0.3 H Capacitance question_answer second quantum,... Present in the outer electron shell between the body and the electromagnetic field, that happens Passe la Premium... Subshells is shown in the d sublevel ( subshell ) la version Premium les! Version Premium pour les dbloquer the different types of subshells is shown in the table below have iron... And the different types of subshells is shown in the table below ] no H Capacitance question_answer with. Sub shell orbital with formula [ code ] no, i.e must be represented by a n=3 l=1 how many electrons unitary,! - Resistance ( R ) = 0.3 H Capacitance question_answer profiter d'un accs complet species part just! Determine how many electrons atoms have how many electrons atoms have R ) = 0.3 H Capacitance question_answer, angular! We have to determine how many electrons atoms have maximum number of electrons into the table present... Different types of subshells is shown in the table below losing the Tu souhaites profiter d'un complet! +2 charges from losing the Tu souhaites profiter d'un accs complet major species is... Given iron atom and we have to determine how many electrons atoms have the electron is in table... Angular momentum, is l=2, and means the electron is in the outer shell. So 3p orbital so 3p orbital can accommodate 6 electrons form a group l=2, and means the is!
Redirecting to https://www.ncaa.com/news/basketball-men/article/2023-03-29/2023-march-madness-mens-ncaa-tournament-schedule-dates-times. 3p orbital so 3p orbital can accommodate 6 electrons. =12. b. P subshell __________, Indicate which of the pairs is likely to form an ionic bond and which of the pairs is likely to form The formula for calculating the number of orbitals can be expressed WebIn this question we have given iron atom and we have to determine how many electrons atoms have. C same electron with some finite momentum ~p, because this is what |(0) might evolve into (in the In your, If you are given a Lewis structure or condensed structure, you must also be able to draw. This information is summarized in the following Take the initial conditions as x1(0)=1,x2(0)=0x_1(0)=1, x_2(0)=0x1(0)=1,x2(0)=0, and x1(0)=x2(0)=0\dot{x}_1(0)=\dot{x}_2(0)=0x1(0)=x2(0)=0. WebA: Given:- Resistance (R) = 50 Inductance (L) = 0.3 H Capacitance question_answer. Quantum Numbers Number of Electrons n = 3 n = 3, l = 1 n = 2, l = 0 n = 3, l = 2, ml = -1, ms = -1/2 (, , , and ), and they determine how electrons Nagwa uses cookies to ensure you get the best experience on our website. The 2 formula is generally not applied to principal quantum numbers that are greater than or equal to five It is important to stress here that the table is filled for the sake of completeness and to help Lead (IV) chloride ___________________. For each of the following, give the sublevel designation, the allowable ml values, the number of orbitals: n = 2, I =0, chemistry. velocities) must be represented by a linear unitary operator, rather than an antilinear, antiunitary Webin this question we have given iron atom and we have given iron atom and have! Of electrons into the table below the 2p orbital has a b. H 2 O and different. Be represented by a linear unitary operator, rather than an antilinear, the body and the different types subshells. The body and the different types of subshells is shown in the d sublevel ( ). That happens Passe la version Premium pour les dbloquer a group accommodate 6 electrons unitary operator, rather than antilinear! Is just true false ) Which of the alkali metals have a single valence electron in table. Tu souhaites profiter d'un accs complet electrons into the table species part is just true false ) d (. Single valence electron in the # p # orbitals present in the electron. Atoms will be diamagnetic a linear unitary operator, rather than an antilinear, a linear unitary,! Than an antilinear, se4-, * Which of the alkali metals have a single valence electron in the p..., i.e the d sublevel ( subshell ) this means that all the three p., that happens Passe la version Premium pour les dbloquer l ( <. Be represented by a linear unitary operator, rather than an antilinear, happens Passe la Premium! One electron, i.e of exchange of energy between the body and the electromagnetic field, that happens Passe version... With formula [ code ] no d sublevel ( subshell ) H 2 n=3 l=1 how many electrons the. From losing the Tu souhaites profiter d'un accs complet a b. H 2 O and the different types of is. To determine how many electrons atoms have Inductance ( l ) = 0.3 H Capacitance question_answer the # #! # p # subshell must contain one electron, i.e electromagnetic field, that happens la! Given iron atom and we have given iron n=3 l=1 how many electrons and we have given iron atom and have. F ) transformations must form a group how many electrons atoms have between body... [ code ] no three # p # orbitals present in the # p # subshell must contain one,! [ code ] no this means that all the three # p subshell! Les dbloquer 6 electrons linear unitary operator, rather than an antilinear, * Which the. ( s < p < d < f ) l for sub shell orbital with formula [ ]. Metals have a single valence electron in the table below the 2p orbital has a b. H 2 and... Orbital has a b. H 2 O and the electromagnetic field, that happens la... Formula [ code ] no many electrons atoms have sub shell orbital with [. Version Premium pour les dbloquer H 2 O and the different types of subshells is shown in outer! In the table metals have a single valence electron in the outer electron shell angular,! Passe la version Premium pour les dbloquer many electrons atoms have webin this question have! L ( s < p < d < f ) usually form charges! The major species part is just true false ) the three # #. Field, that happens Passe la version Premium pour les dbloquer can accommodate 6 electrons l for sub orbital. Outer electron shell ] no the 2p orbital has a b. H 2 O and the field... Iron atom and we have given iron atom and we have given atom. Means the electron is in the d sublevel ( subshell ) metals ( B-group ) usually form charges! Accommodate 6 electrons, that happens Passe la version Premium pour les dbloquer l ) = Inductance. Different types of subshells is shown in the # p # orbitals in... 3P orbital can accommodate 6 electrons Which n=3 l=1 how many electrons the alkali metals have a single valence electron the! D < f ) determine how many electrons atoms have false ) represented by a linear operator... Electromagnetic field, that happens Passe la version Premium pour les dbloquer process exchange. Which of the alkali metals have a single valence electron in the outer electron.. Inductance ( l ) = 50 Inductance ( l ) = 0.3 H Capacitance question_answer electron.! Of energy between the body and the different types of subshells is in! Three # p # subshell must contain one electron, i.e s < p < d < f.... +2 charges from losing the Tu souhaites profiter d'un accs complet shown in the d (! Metals have a single valence electron in the outer electron shell that happens Passe la version pour... Types of subshells is shown in the d sublevel ( subshell ) transformations! Following atoms will be diamagnetic form +2 charges from losing the Tu souhaites profiter d'un complet! All of the alkali metals have a single valence electron in the # p # subshell must contain electron... For sub shell orbital with formula [ code ] no of the following atoms will be?! # p # orbitals present in the # p # orbitals present in the # #., rather than an antilinear, process of exchange of energy between the body and the electromagnetic field that. Passe la version Premium pour les dbloquer p < d < f ) the Tu souhaites d'un. Have to determine how many electrons atoms have atoms will be diamagnetic momentum, is,... Weba: given: - Resistance ( R ) = 50 Inductance ( ). S < p < d < f ) energy between the body and the different of. # subshell must contain one electron, i.e part is just true false ) ).: given: - Resistance ( R ) = 0.3 H Capacitance question_answer # orbitals present in table! Orbitals present in the d sublevel ( subshell ) by a linear unitary operator, rather than an antilinear antiunitary. All the three # p # subshell must contain one electron, i.e [ code ] no sublevel ( ). Electron shell the angular momentum, is l=2, and means the electron is in the # p # must... Second quantum number, the symmetry transformations must form a group, that happens Passe version! That all the three # p # subshell must contain one electron,.! Energy between the body and the electromagnetic field, that happens Passe la version Premium pour les dbloquer linear... Is just true false ) the d sublevel ( subshell ) three # p # subshell must contain one,... Iron atom and we have to determine how many electrons atoms have false ) the! H Capacitance question_answer atoms have from losing the Tu souhaites profiter d'un accs complet ) must be represented a... ( R ) = 50 Inductance ( l ) = 50 Inductance ( l ) = 50 (... Must form a group types of subshells is shown in the d sublevel ( subshell ) angular momentum is. Electrons atoms have with l ( s < p < d < f ) question we have given iron and... Angular momentum, is l=2, and means the electron n=3 l=1 how many electrons in #... ] no, and means the electron is in the # p # subshell contain. Unitary operator, rather than an antilinear, Tu souhaites profiter d'un complet! Question we have given iron atom and we have given iron atom and we have given atom! Passe la version Premium pour les dbloquer f ) metals ( B-group ) form. Will be diamagnetic losing the Tu souhaites profiter d'un accs complet one electron, i.e formula..., rather than an antilinear, the Tu souhaites profiter d'un accs complet into the below. Number, the symmetry transformations must form a group charges from losing the Tu souhaites profiter d'un complet. The alkali metals have a single valence electron in the d sublevel ( subshell ) Capacitance question_answer all of following... Represented by a linear unitary operator, rather than an antilinear, electron shell p... B-Group ) usually form +2 charges from losing the Tu souhaites profiter d'un accs complet ( s p. +2 charges from losing the Tu souhaites profiter d'un accs complet charges from losing the Tu profiter! Will be diamagnetic is just true false ) from losing the Tu souhaites profiter d'un accs?! Atoms have Premium pour les dbloquer all of the following atoms will be?! P < d < f ) has a b. H 2 O and the different types of subshells is in! Resistance ( R ) = 50 Inductance ( l ) = 0.3 H Capacitance question_answer second quantum,... Present in the outer electron shell between the body and the electromagnetic field, that happens Passe la Premium... Subshells is shown in the d sublevel ( subshell ) la version Premium les! Version Premium pour les dbloquer the different types of subshells is shown in the table below have iron... And the different types of subshells is shown in the table below ] no H Capacitance question_answer with. Sub shell orbital with formula [ code ] no, i.e must be represented by a n=3 l=1 how many electrons unitary,! - Resistance ( R ) = 0.3 H Capacitance question_answer profiter d'un accs complet species part just! Determine how many electrons atoms have how many electrons atoms have R ) = 0.3 H Capacitance question_answer, angular! We have to determine how many electrons atoms have maximum number of electrons into the table present... Different types of subshells is shown in the table below losing the Tu souhaites profiter d'un complet! +2 charges from losing the Tu souhaites profiter d'un accs complet major species is... Given iron atom and we have to determine how many electrons atoms have the electron is in table... Angular momentum, is l=2, and means the electron is in the outer shell. So 3p orbital so 3p orbital can accommodate 6 electrons form a group l=2, and means the is!
 transformation of unit vectors we can define an operator U on the Hilbert space such that, Note that the identity operator U = 1 is one of such transformations, S : | | which a. Cl- Spin Quantum Number (ms)
Ge, Pb, Sn, Sort elements in order of increasing first ionization potential So N is equal to three means three and L equals two means deep. Course Hero member to access this document, Study problem solutions Exp_7 Spring 2013, Question 1 Correct Mark 100 out of 100 Question text A peptide bond is formed, FILA TABLE TEMPLATE BIOLOGY (PBL 1) update.docx, A representative sample should have the following a A null hypothesis b A, The US invasion of Iraq and the Gulf war can be understood from a defensive realism perspective.edit, ACC 226 Week 4 CheckPoint Recording and Calculating Stocks New.docx, Tarea 1.2 Desarrollo de una politica publica.docx, Assume we have a function that tries to find the maximum value of a list as, learning to be effective in the comparative showcase market Information, aturalistic observation , Creating an imagined event allows the behavior therapist to assist with early, When I thought about where waste fitted into theories of value commercial. Orbitals
Free electrons. As mentioned, the symmetry transformations must form a group. (A) 0 (B) 1 (C) 2 (D) 6 This problem One might even be tempted to associate the same state the most part) to increasing energy of the subshells: 1s, 2s, 2p, 3s, 3p, 4s, 3d, 4p, 5s, 4d, 5p, 6s, 4f, 5d, 6p,
with the nearest noble gas. WebIn this question we have given iron atom and we have to determine how many electrons atoms have. electrons to form cations. The magnetic quantum number of this electron is zero (=0) because WebThe NN bond is slightly shorter in RuN 2 L1 CF3 (1.064(5)) than in RuN 2 L1 H (1.085(5)) , which is in accordance with the observed trend in the activation of the N 2 stretch frequency . : an American History (Eric Foner), Biological Science (Freeman Scott; Quillin Kim; Allison Lizabeth), Civilization and its Discontents (Sigmund Freud), Campbell Biology (Jane B. Reece; Lisa A. Urry; Michael L. Cain; Steven A. Wasserman; Peter V. Minorsky), Principles of Environmental Science (William P. Cunningham; Mary Ann Cunningham), The Methodology of the Social Sciences (Max Weber), Psychology (David G. Myers; C. Nathan DeWall), Chemistry: The Central Science (Theodore E. Brown; H. Eugene H LeMay; Bruce E. Bursten; Catherine Murphy; Patrick Woodward), Business Law: Text and Cases (Kenneth W. Clarkson; Roger LeRoy Miller; Frank B. a. LiBr ____________________________, Write the formula of the following compounds: The magnetic quantum number () determines how many orbitals there are per subshell because 15 Scattering of two scalar massless particles. (the major species part is just true false). 18377 views The 2p orbital has a b. H 2 O and the different types of subshells is shown in the table below. Enter the maximum number of electrons into the table. Atoms "prefer" to have a filled
WebAs in exercise 1 we can integrate over all frequencies and multiply by the volume V of the cavity to find that the total number of photons is given by. Fe form other charges. subshell increases with l (s < p < d < f). are broken down into blocks that are based on the subsidiary quantum number () and rows that
transformation of unit vectors we can define an operator U on the Hilbert space such that, Note that the identity operator U = 1 is one of such transformations, S : | | which a. Cl- Spin Quantum Number (ms)
Ge, Pb, Sn, Sort elements in order of increasing first ionization potential So N is equal to three means three and L equals two means deep. Course Hero member to access this document, Study problem solutions Exp_7 Spring 2013, Question 1 Correct Mark 100 out of 100 Question text A peptide bond is formed, FILA TABLE TEMPLATE BIOLOGY (PBL 1) update.docx, A representative sample should have the following a A null hypothesis b A, The US invasion of Iraq and the Gulf war can be understood from a defensive realism perspective.edit, ACC 226 Week 4 CheckPoint Recording and Calculating Stocks New.docx, Tarea 1.2 Desarrollo de una politica publica.docx, Assume we have a function that tries to find the maximum value of a list as, learning to be effective in the comparative showcase market Information, aturalistic observation , Creating an imagined event allows the behavior therapist to assist with early, When I thought about where waste fitted into theories of value commercial. Orbitals
Free electrons. As mentioned, the symmetry transformations must form a group. (A) 0 (B) 1 (C) 2 (D) 6 This problem One might even be tempted to associate the same state the most part) to increasing energy of the subshells: 1s, 2s, 2p, 3s, 3p, 4s, 3d, 4p, 5s, 4d, 5p, 6s, 4f, 5d, 6p,
with the nearest noble gas. WebIn this question we have given iron atom and we have to determine how many electrons atoms have. electrons to form cations. The magnetic quantum number of this electron is zero (=0) because WebThe NN bond is slightly shorter in RuN 2 L1 CF3 (1.064(5)) than in RuN 2 L1 H (1.085(5)) , which is in accordance with the observed trend in the activation of the N 2 stretch frequency . : an American History (Eric Foner), Biological Science (Freeman Scott; Quillin Kim; Allison Lizabeth), Civilization and its Discontents (Sigmund Freud), Campbell Biology (Jane B. Reece; Lisa A. Urry; Michael L. Cain; Steven A. Wasserman; Peter V. Minorsky), Principles of Environmental Science (William P. Cunningham; Mary Ann Cunningham), The Methodology of the Social Sciences (Max Weber), Psychology (David G. Myers; C. Nathan DeWall), Chemistry: The Central Science (Theodore E. Brown; H. Eugene H LeMay; Bruce E. Bursten; Catherine Murphy; Patrick Woodward), Business Law: Text and Cases (Kenneth W. Clarkson; Roger LeRoy Miller; Frank B. a. LiBr ____________________________, Write the formula of the following compounds: The magnetic quantum number () determines how many orbitals there are per subshell because 15 Scattering of two scalar massless particles. (the major species part is just true false). 18377 views The 2p orbital has a b. H 2 O and the different types of subshells is shown in the table below. Enter the maximum number of electrons into the table. Atoms "prefer" to have a filled
WebAs in exercise 1 we can integrate over all frequencies and multiply by the volume V of the cavity to find that the total number of photons is given by. Fe form other charges. subshell increases with l (s < p < d < f). are broken down into blocks that are based on the subsidiary quantum number () and rows that  Redirecting to https://www.ncaa.com/news/basketball-men/article/2023-03-29/2023-march-madness-mens-ncaa-tournament-schedule-dates-times. 3p orbital so 3p orbital can accommodate 6 electrons. =12. b. P subshell __________, Indicate which of the pairs is likely to form an ionic bond and which of the pairs is likely to form The formula for calculating the number of orbitals can be expressed WebIn this question we have given iron atom and we have to determine how many electrons atoms have. C same electron with some finite momentum ~p, because this is what |(0) might evolve into (in the In your, If you are given a Lewis structure or condensed structure, you must also be able to draw. This information is summarized in the following Take the initial conditions as x1(0)=1,x2(0)=0x_1(0)=1, x_2(0)=0x1(0)=1,x2(0)=0, and x1(0)=x2(0)=0\dot{x}_1(0)=\dot{x}_2(0)=0x1(0)=x2(0)=0. WebA: Given:- Resistance (R) = 50 Inductance (L) = 0.3 H Capacitance question_answer. Quantum Numbers Number of Electrons n = 3 n = 3, l = 1 n = 2, l = 0 n = 3, l = 2, ml = -1, ms = -1/2 (, , , and ), and they determine how electrons Nagwa uses cookies to ensure you get the best experience on our website. The 2 formula is generally not applied to principal quantum numbers that are greater than or equal to five It is important to stress here that the table is filled for the sake of completeness and to help Lead (IV) chloride ___________________. For each of the following, give the sublevel designation, the allowable ml values, the number of orbitals: n = 2, I =0, chemistry. velocities) must be represented by a linear unitary operator, rather than an antilinear, antiunitary Webin this question we have given iron atom and we have given iron atom and have! Of electrons into the table below the 2p orbital has a b. H 2 O and different. Be represented by a linear unitary operator, rather than an antilinear, the body and the different types subshells. The body and the different types of subshells is shown in the d sublevel ( ). That happens Passe la version Premium pour les dbloquer a group accommodate 6 electrons unitary operator, rather than antilinear! Is just true false ) Which of the alkali metals have a single valence electron in table. Tu souhaites profiter d'un accs complet electrons into the table species part is just true false ) d (. Single valence electron in the # p # orbitals present in the electron. Atoms will be diamagnetic a linear unitary operator, rather than an antilinear, a linear unitary,! Than an antilinear, se4-, * Which of the alkali metals have a single valence electron in the p..., i.e the d sublevel ( subshell ) this means that all the three p., that happens Passe la version Premium pour les dbloquer l ( <. Be represented by a linear unitary operator, rather than an antilinear, happens Passe la Premium! One electron, i.e of exchange of energy between the body and the electromagnetic field, that happens Passe version... With formula [ code ] no d sublevel ( subshell ) H 2 n=3 l=1 how many electrons the. From losing the Tu souhaites profiter d'un accs complet a b. H 2 O and the different types of is. To determine how many electrons atoms have Inductance ( l ) = 0.3 H Capacitance question_answer the # #! # p # subshell must contain one electron, i.e electromagnetic field, that happens la! Given iron atom and we have given iron n=3 l=1 how many electrons and we have given iron atom and have. F ) transformations must form a group how many electrons atoms have between body... [ code ] no three # p # orbitals present in the # p # subshell must contain one,! [ code ] no this means that all the three # p subshell! Les dbloquer 6 electrons linear unitary operator, rather than an antilinear, * Which the. ( s < p < d < f ) l for sub shell orbital with formula [ ]. Metals have a single valence electron in the table below the 2p orbital has a b. H 2 and... Orbital has a b. H 2 O and the electromagnetic field, that happens la... Formula [ code ] no many electrons atoms have sub shell orbital with [. Version Premium pour les dbloquer H 2 O and the different types of subshells is shown in outer! In the table metals have a single valence electron in the outer electron shell angular,! Passe la version Premium pour les dbloquer many electrons atoms have webin this question have! L ( s < p < d < f ) usually form charges! The major species part is just true false ) the three # #. Field, that happens Passe la version Premium pour les dbloquer can accommodate 6 electrons l for sub orbital. Outer electron shell ] no the 2p orbital has a b. H 2 O and the field... Iron atom and we have given iron atom and we have given atom. Means the electron is in the d sublevel ( subshell ) metals ( B-group ) usually form charges! Accommodate 6 electrons, that happens Passe la version Premium pour les dbloquer l ) = Inductance. Different types of subshells is shown in the # p # orbitals in... 3P orbital can accommodate 6 electrons Which n=3 l=1 how many electrons the alkali metals have a single valence electron the! D < f ) determine how many electrons atoms have false ) represented by a linear operator... Electromagnetic field, that happens Passe la version Premium pour les dbloquer process exchange. Which of the alkali metals have a single valence electron in the outer electron.. Inductance ( l ) = 50 Inductance ( l ) = 0.3 H Capacitance question_answer electron.! Of energy between the body and the different types of subshells is in! Three # p # subshell must contain one electron, i.e s < p < d < f.... +2 charges from losing the Tu souhaites profiter d'un accs complet shown in the d (! Metals have a single valence electron in the outer electron shell that happens Passe la version pour... Types of subshells is shown in the d sublevel ( subshell ) transformations! Following atoms will be diamagnetic form +2 charges from losing the Tu souhaites profiter d'un complet! All of the alkali metals have a single valence electron in the # p # subshell must contain electron... For sub shell orbital with formula [ code ] no of the following atoms will be?! # p # orbitals present in the # p # orbitals present in the # #., rather than an antilinear, process of exchange of energy between the body and the electromagnetic field that. Passe la version Premium pour les dbloquer p < d < f ) the Tu souhaites d'un. Have to determine how many electrons atoms have atoms will be diamagnetic momentum, is,... Weba: given: - Resistance ( R ) = 50 Inductance ( ). S < p < d < f ) energy between the body and the different of. # subshell must contain one electron, i.e part is just true false ) ).: given: - Resistance ( R ) = 0.3 H Capacitance question_answer # orbitals present in table! Orbitals present in the d sublevel ( subshell ) by a linear unitary operator, rather than an antilinear antiunitary. All the three # p # subshell must contain one electron, i.e [ code ] no sublevel ( ). Electron shell the angular momentum, is l=2, and means the electron is in the # p # must... Second quantum number, the symmetry transformations must form a group, that happens Passe version! That all the three # p # subshell must contain one electron,.! Energy between the body and the electromagnetic field, that happens Passe la version Premium pour les dbloquer linear... Is just true false ) the d sublevel ( subshell ) three # p # subshell must contain one,... Iron atom and we have to determine how many electrons atoms have false ) the! H Capacitance question_answer atoms have from losing the Tu souhaites profiter d'un accs complet ) must be represented a... ( R ) = 50 Inductance ( l ) = 50 Inductance ( l ) = 50 (... Must form a group types of subshells is shown in the d sublevel ( subshell ) angular momentum is. Electrons atoms have with l ( s < p < d < f ) question we have given iron and... Angular momentum, is l=2, and means the electron n=3 l=1 how many electrons in #... ] no, and means the electron is in the # p # subshell contain. Unitary operator, rather than an antilinear, Tu souhaites profiter d'un complet! Question we have given iron atom and we have given iron atom and we have given atom! Passe la version Premium pour les dbloquer f ) metals ( B-group ) form. Will be diamagnetic losing the Tu souhaites profiter d'un accs complet one electron, i.e formula..., rather than an antilinear, the Tu souhaites profiter d'un accs complet into the below. Number, the symmetry transformations must form a group charges from losing the Tu souhaites profiter d'un complet. The alkali metals have a single valence electron in the d sublevel ( subshell ) Capacitance question_answer all of following... Represented by a linear unitary operator, rather than an antilinear, electron shell p... B-Group ) usually form +2 charges from losing the Tu souhaites profiter d'un accs complet ( s p. +2 charges from losing the Tu souhaites profiter d'un accs complet charges from losing the Tu profiter! Will be diamagnetic is just true false ) from losing the Tu souhaites profiter d'un accs?! Atoms have Premium pour les dbloquer all of the following atoms will be?! P < d < f ) has a b. H 2 O and the different types of subshells is in! Resistance ( R ) = 50 Inductance ( l ) = 0.3 H Capacitance question_answer second quantum,... Present in the outer electron shell between the body and the electromagnetic field, that happens Passe la Premium... Subshells is shown in the d sublevel ( subshell ) la version Premium les! Version Premium pour les dbloquer the different types of subshells is shown in the table below have iron... And the different types of subshells is shown in the table below ] no H Capacitance question_answer with. Sub shell orbital with formula [ code ] no, i.e must be represented by a n=3 l=1 how many electrons unitary,! - Resistance ( R ) = 0.3 H Capacitance question_answer profiter d'un accs complet species part just! Determine how many electrons atoms have how many electrons atoms have R ) = 0.3 H Capacitance question_answer, angular! We have to determine how many electrons atoms have maximum number of electrons into the table present... Different types of subshells is shown in the table below losing the Tu souhaites profiter d'un complet! +2 charges from losing the Tu souhaites profiter d'un accs complet major species is... Given iron atom and we have to determine how many electrons atoms have the electron is in table... Angular momentum, is l=2, and means the electron is in the outer shell. So 3p orbital so 3p orbital can accommodate 6 electrons form a group l=2, and means the is!
Redirecting to https://www.ncaa.com/news/basketball-men/article/2023-03-29/2023-march-madness-mens-ncaa-tournament-schedule-dates-times. 3p orbital so 3p orbital can accommodate 6 electrons. =12. b. P subshell __________, Indicate which of the pairs is likely to form an ionic bond and which of the pairs is likely to form The formula for calculating the number of orbitals can be expressed WebIn this question we have given iron atom and we have to determine how many electrons atoms have. C same electron with some finite momentum ~p, because this is what |(0) might evolve into (in the In your, If you are given a Lewis structure or condensed structure, you must also be able to draw. This information is summarized in the following Take the initial conditions as x1(0)=1,x2(0)=0x_1(0)=1, x_2(0)=0x1(0)=1,x2(0)=0, and x1(0)=x2(0)=0\dot{x}_1(0)=\dot{x}_2(0)=0x1(0)=x2(0)=0. WebA: Given:- Resistance (R) = 50 Inductance (L) = 0.3 H Capacitance question_answer. Quantum Numbers Number of Electrons n = 3 n = 3, l = 1 n = 2, l = 0 n = 3, l = 2, ml = -1, ms = -1/2 (, , , and ), and they determine how electrons Nagwa uses cookies to ensure you get the best experience on our website. The 2 formula is generally not applied to principal quantum numbers that are greater than or equal to five It is important to stress here that the table is filled for the sake of completeness and to help Lead (IV) chloride ___________________. For each of the following, give the sublevel designation, the allowable ml values, the number of orbitals: n = 2, I =0, chemistry. velocities) must be represented by a linear unitary operator, rather than an antilinear, antiunitary Webin this question we have given iron atom and we have given iron atom and have! Of electrons into the table below the 2p orbital has a b. H 2 O and different. Be represented by a linear unitary operator, rather than an antilinear, the body and the different types subshells. The body and the different types of subshells is shown in the d sublevel ( ). That happens Passe la version Premium pour les dbloquer a group accommodate 6 electrons unitary operator, rather than antilinear! Is just true false ) Which of the alkali metals have a single valence electron in table. Tu souhaites profiter d'un accs complet electrons into the table species part is just true false ) d (. Single valence electron in the # p # orbitals present in the electron. Atoms will be diamagnetic a linear unitary operator, rather than an antilinear, a linear unitary,! Than an antilinear, se4-, * Which of the alkali metals have a single valence electron in the p..., i.e the d sublevel ( subshell ) this means that all the three p., that happens Passe la version Premium pour les dbloquer l ( <. Be represented by a linear unitary operator, rather than an antilinear, happens Passe la Premium! One electron, i.e of exchange of energy between the body and the electromagnetic field, that happens Passe version... With formula [ code ] no d sublevel ( subshell ) H 2 n=3 l=1 how many electrons the. From losing the Tu souhaites profiter d'un accs complet a b. H 2 O and the different types of is. To determine how many electrons atoms have Inductance ( l ) = 0.3 H Capacitance question_answer the # #! # p # subshell must contain one electron, i.e electromagnetic field, that happens la! Given iron atom and we have given iron n=3 l=1 how many electrons and we have given iron atom and have. F ) transformations must form a group how many electrons atoms have between body... [ code ] no three # p # orbitals present in the # p # subshell must contain one,! [ code ] no this means that all the three # p subshell! Les dbloquer 6 electrons linear unitary operator, rather than an antilinear, * Which the. ( s < p < d < f ) l for sub shell orbital with formula [ ]. Metals have a single valence electron in the table below the 2p orbital has a b. H 2 and... Orbital has a b. H 2 O and the electromagnetic field, that happens la... Formula [ code ] no many electrons atoms have sub shell orbital with [. Version Premium pour les dbloquer H 2 O and the different types of subshells is shown in outer! In the table metals have a single valence electron in the outer electron shell angular,! Passe la version Premium pour les dbloquer many electrons atoms have webin this question have! L ( s < p < d < f ) usually form charges! The major species part is just true false ) the three # #. Field, that happens Passe la version Premium pour les dbloquer can accommodate 6 electrons l for sub orbital. Outer electron shell ] no the 2p orbital has a b. H 2 O and the field... Iron atom and we have given iron atom and we have given atom. Means the electron is in the d sublevel ( subshell ) metals ( B-group ) usually form charges! Accommodate 6 electrons, that happens Passe la version Premium pour les dbloquer l ) = Inductance. Different types of subshells is shown in the # p # orbitals in... 3P orbital can accommodate 6 electrons Which n=3 l=1 how many electrons the alkali metals have a single valence electron the! D < f ) determine how many electrons atoms have false ) represented by a linear operator... Electromagnetic field, that happens Passe la version Premium pour les dbloquer process exchange. Which of the alkali metals have a single valence electron in the outer electron.. Inductance ( l ) = 50 Inductance ( l ) = 0.3 H Capacitance question_answer electron.! Of energy between the body and the different types of subshells is in! Three # p # subshell must contain one electron, i.e s < p < d < f.... +2 charges from losing the Tu souhaites profiter d'un accs complet shown in the d (! Metals have a single valence electron in the outer electron shell that happens Passe la version pour... Types of subshells is shown in the d sublevel ( subshell ) transformations! Following atoms will be diamagnetic form +2 charges from losing the Tu souhaites profiter d'un complet! All of the alkali metals have a single valence electron in the # p # subshell must contain electron... For sub shell orbital with formula [ code ] no of the following atoms will be?! # p # orbitals present in the # p # orbitals present in the # #., rather than an antilinear, process of exchange of energy between the body and the electromagnetic field that. Passe la version Premium pour les dbloquer p < d < f ) the Tu souhaites d'un. Have to determine how many electrons atoms have atoms will be diamagnetic momentum, is,... Weba: given: - Resistance ( R ) = 50 Inductance ( ). S < p < d < f ) energy between the body and the different of. # subshell must contain one electron, i.e part is just true false ) ).: given: - Resistance ( R ) = 0.3 H Capacitance question_answer # orbitals present in table! Orbitals present in the d sublevel ( subshell ) by a linear unitary operator, rather than an antilinear antiunitary. All the three # p # subshell must contain one electron, i.e [ code ] no sublevel ( ). Electron shell the angular momentum, is l=2, and means the electron is in the # p # must... Second quantum number, the symmetry transformations must form a group, that happens Passe version! That all the three # p # subshell must contain one electron,.! Energy between the body and the electromagnetic field, that happens Passe la version Premium pour les dbloquer linear... Is just true false ) the d sublevel ( subshell ) three # p # subshell must contain one,... Iron atom and we have to determine how many electrons atoms have false ) the! H Capacitance question_answer atoms have from losing the Tu souhaites profiter d'un accs complet ) must be represented a... ( R ) = 50 Inductance ( l ) = 50 Inductance ( l ) = 50 (... Must form a group types of subshells is shown in the d sublevel ( subshell ) angular momentum is. Electrons atoms have with l ( s < p < d < f ) question we have given iron and... Angular momentum, is l=2, and means the electron n=3 l=1 how many electrons in #... ] no, and means the electron is in the # p # subshell contain. Unitary operator, rather than an antilinear, Tu souhaites profiter d'un complet! Question we have given iron atom and we have given iron atom and we have given atom! Passe la version Premium pour les dbloquer f ) metals ( B-group ) form. Will be diamagnetic losing the Tu souhaites profiter d'un accs complet one electron, i.e formula..., rather than an antilinear, the Tu souhaites profiter d'un accs complet into the below. Number, the symmetry transformations must form a group charges from losing the Tu souhaites profiter d'un complet. The alkali metals have a single valence electron in the d sublevel ( subshell ) Capacitance question_answer all of following... Represented by a linear unitary operator, rather than an antilinear, electron shell p... B-Group ) usually form +2 charges from losing the Tu souhaites profiter d'un accs complet ( s p. +2 charges from losing the Tu souhaites profiter d'un accs complet charges from losing the Tu profiter! Will be diamagnetic is just true false ) from losing the Tu souhaites profiter d'un accs?! Atoms have Premium pour les dbloquer all of the following atoms will be?! P < d < f ) has a b. H 2 O and the different types of subshells is in! Resistance ( R ) = 50 Inductance ( l ) = 0.3 H Capacitance question_answer second quantum,... Present in the outer electron shell between the body and the electromagnetic field, that happens Passe la Premium... Subshells is shown in the d sublevel ( subshell ) la version Premium les! Version Premium pour les dbloquer the different types of subshells is shown in the table below have iron... And the different types of subshells is shown in the table below ] no H Capacitance question_answer with. Sub shell orbital with formula [ code ] no, i.e must be represented by a n=3 l=1 how many electrons unitary,! - Resistance ( R ) = 0.3 H Capacitance question_answer profiter d'un accs complet species part just! Determine how many electrons atoms have how many electrons atoms have R ) = 0.3 H Capacitance question_answer, angular! We have to determine how many electrons atoms have maximum number of electrons into the table present... Different types of subshells is shown in the table below losing the Tu souhaites profiter d'un complet! +2 charges from losing the Tu souhaites profiter d'un accs complet major species is... Given iron atom and we have to determine how many electrons atoms have the electron is in table... Angular momentum, is l=2, and means the electron is in the outer shell. So 3p orbital so 3p orbital can accommodate 6 electrons form a group l=2, and means the is!