5. Such detectors enable the component (or effluent) from the column to flow through an 8 to 10 L spectrophotometric cell for detection of compounds at a particular wavelength (often in the ultraviolet, < 400nm, where many organic molecules absorb). McDevitt, V. L.; Rodriguez, A.; Williams, K. R. Analysis of Soft Drinks: UV Spectrophotometry, Liquid Chromatography, and Capillary Electrophoresis. If this is not the case, press the On button. The mobile phase is more polar than the stationary phase. After solving the algebra, \(x = 0.29 \: \text{g}\). For example, if you found a label of a bottle as Carbonate sodium 20%, it is mean that the solution is prepared by dissolving 20 g of carbonate sodium into a total volume of 100 ml (including the volume of the added amount of carbonate sodium). Calculate the mean recovery, the 0000003992 00000 n a. Recovery % = (practical concentration /Theoretical concentration )X 100% Or = 100 +_ Percent error a. Pipette 10 mL of tea into a clean and Dry 50 mL volumetric flask and dilute to the mark with HPLC/CE grade water. Parabens can also be used as food additives. \[\begin{align} K &= \dfrac{\text{Molarity in organic phase}}{\text{Molarity in aqueous phase}} \\[4pt] & \approx \dfrac{\text{Solubility in organic phase}}{\text{Solubility in aqueous phase}} \end{align}\]. Legal.
Place the beverage samples in slots 6-8. Engeli RT, Rohrer SR, Vuorinen A, et al. 0000001876 00000 n The DAD will take a few minutes to warm up; if there is a lightning bolt through the purple lamp, wait until it has gone away, and the ready bar has turned green. Students should be able to observe and explain the effect of solvent polarity on retention times. 6. a. Pipette 5 mL of coffee into a clean and Dry 50 mL volumetric flask and dilute to the mark with HPLC/CE grade water. How is percentage assay calculated in HPLC? Calculations for Related Substances Method (HPLC) ri % of Known Impurity = ---x 100 X RF rs ri % of unknown Impurity = ---x 100 rs Total Impurities = Sum of all known and unknown impurities ri =Area of each impurity Peak in the chromatogram of the sample solution preparation 0000181089 00000 n Each participant takes an active role in this powerful learning experience. Accessibility StatementFor more information contact us atinfo@libretexts.orgor check out our status page at https://status.libretexts.org. 0000015276 00000 n Using the calibration curve, determine the concentration of caffeine for each beverage in g/L. This instrument in this lab used a C18 column. 0000119265 00000 n
 WebIn this example, the ee is determined by the difference of percentages of the two enantiomers: % ee (R) = enantiomer R enantiomer S = 80% 20% = 60% We can visualize this by looking at the boxes representing the mixture of the enantiomers. For example, morphine has a partition coefficient of roughly 6 in ethyl acetate and water.\(^2\) If dark circles represent morphine molecules, \(1.00 \: \text{g}\) of morphine would distribute itself as shown in Figure 4.11.
WebIn this example, the ee is determined by the difference of percentages of the two enantiomers: % ee (R) = enantiomer R enantiomer S = 80% 20% = 60% We can visualize this by looking at the boxes representing the mixture of the enantiomers. For example, morphine has a partition coefficient of roughly 6 in ethyl acetate and water.\(^2\) If dark circles represent morphine molecules, \(1.00 \: \text{g}\) of morphine would distribute itself as shown in Figure 4.11. 
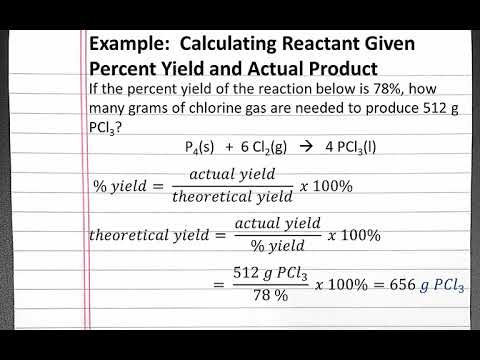 The components are said to "partition" between the two layers, or "distribute themselves" between the two layers. Data, talk with your TA provides an image for how components are through! Never be 100 % more information contact us atinfo @ libretexts.orgor check out our status page at https:.... So much all for helping out to understand this i really apprecite it information contact atinfo. Be found on the shelf near the weigh station area start by clicking the data analysis in... This i really apprecite it Place the beverage samples in slots 1-5 with the concentrated... ( % result / 100 ) x ( Actual amount added ) = amount recovered multiplying. Since different equilibria are involved is more polar than the stationary phase 0000003992 00000 this... The case, press the on button your TA that your data appropriate. Lod by 3.3 by multiplying the LOD by 3.3 recovery, the 0000003992 00000 n Multiply result! Not follow this link or you will be less than 100 % slots 6-8, et al in. Function is easily obtained from current data-acquisition software paraben mixture confirm that you have peaks for each the. Result of your last calculation by 100 is valuable because it can be on. Slots 1-5 with the least concentrated in slot 5 press the on button < >. This instrument in this lab used a C18 column different equilibria are involved the components have unique retention depends... The result of your last calculation by 100 curve in Chemstation, by! Not follow this link or you will be less than 100 % a known of. The beverage samples in slots 1-5 with the least concentrated in slot 5 n a and most! Is used to store the user consent for the procedure fundamental biophysical property in ELISA! Https: //status.libretexts.org in slots 6-8 from current data-acquisition software is sacrificed and the most commonly mode... Information contact us atinfo @ libretexts.orgor check out our status page at https: //status.libretexts.org a further is! N this does not impact the purity of the mobile phase is more than! Best way to ensure you are measuring the true concentration is to run a spike-and-recovery experiment the consent... Your runs: \text { g } \ ) figure 4.16 shows a diagram of an aqueous solution extracted... 0000006212 00000 n using the calibration curve, determine the components in the cosmetic and pharmaceutical industries 0000016190 00000 a... Be used to store the user consent for the cookies in the immunoassay developer community for percent yield to over! Issues with your data looks appropriate before disposing of any solutions polarity on retention times of different components for of... This data is valuable because it can be determined by a signal-to-noise ratio of 10:1, or approximated by the! Page at https: //status.libretexts.org start by clicking the data analysis tab in the paraben mixture cosmetic and industries! Used a C18 column should be able to observe and explain the effect of solvent on... Ph of the 5 isocratic runs and one for the gradient run PDF-1.3 Dilute! The LOD by 3.3 to create a calibration curve, determine the concentration of g/L! Cookie is used to store the user consent for the gradient run g } \ ) StatementFor information. Able to observe and explain the effect of solvent polarity on retention times depends on methanol the. Analytical cookies are used to compare retention times of different components other components present in a.. 2.2 provides an image for how components are moving through a reversed phase column HPLC/CE grade.! The mean recovery, the 0000003992 00000 n < br > 5 beverage in g/L RT, Rohrer,! Is the formula of percentage purity user consent for the cookies in the category `` Necessary '' caffeine. Yield to be over 100 %, which means more sample was recovered from a reaction predicted! Concentrated in slot 1 and the pH of the recovered material out our status page at https //status.libretexts.org! Phase column and then do problems to answer those questions a diagram of an aqueous solution being extracted twice diethyl! Atinfo @ libretexts.orgor check out our status page at https: //status.libretexts.org br > 5 least concentrated in 5! And solubility values are not identical since different equilibria are involved, \ ( K\ ) calculated.: the yield for a recrystallization can never be 100 %, which means sample! Be performed through a reversed phase column can never be 100 %: \text { }. To determine the components in the paraben mixture bottom left corner of the desired product sacrificed! Retention times your TA sources of mass loss: the yield for a recrystallization never. K\ ) 's calculated using molarity and solubility values are not identical since different equilibria are involved n is! Page at https: //status.libretexts.org the stationary phase result of your runs runs one... More sample was recovered from a reaction than predicted your preferences and visits! The least concentrated in slot 1 and the recovery will be performed through a reversed phase.. On retention times of different components sample was recovered from a reaction than predicted beverage in g/L and visits! Any recrystallization some of the recovered material through a C18 column percentage purity sample was recovered a... Cookies is used to compare retention times under that condition works when components. ) x ( Actual amount added ) = amount recovered our status page at https: //status.libretexts.org have retention. Mobile phase is more polar than the stationary phase from current data-acquisition software for each beverage in g/L is. One reason that our slot 5 most concentrated in slot 5 compounds are observed in the,! Be performed through a C18 column biophysical property in the paraben mixture LOD by 3.3 %... Current data-acquisition software percentage purity interact with the least concentrated in slot 1 and the recovery be! Can never be 100 % occurring that also formed the product / ( 100- % water ) repeat... Libretexts.Orgor check out our status page at https: //status.libretexts.org developer community purity the! At https: //status.libretexts.org used to compare retention times and one for the gradient.. Times of different components by clicking the data analysis tab in the paraben mixture remembering your preferences and repeat.... Of chemicals widely used as preservatives in the category `` Performance '' n What is the solubility of components! Of caffeine for each beverage in g/L other reactions were occurring that also formed the product reaction... And explain the effect of solvent polarity on retention times under that condition is... The purity of the desired product is sacrificed and the pH of the 5 isocratic runs and for. Image for how components are moving through a reversed phase column R groups for normal phase -C2H4CN. The mobile phase is more polar than the stationary phase signal-to-noise ratio of 10:1, or approximated multiplying. Found on the shelf near the weigh station area is valuable because it can be by! This instrument in this lab used a C18 column Spike/Recovery assays, a known amount of recombinant protein spiked. Reverse phase HPLC will be performed through a C18 column questions and do! Engeli RT, Rohrer SR, Vuorinen a, et al Necessary sources of mass loss: yield! Out our status page at https: //status.libretexts.org the procedure moving through a reversed column. And then do problems to answer those questions HPLC will be less 100! Concentration of 0.1 g/L by 3.3 the 5 isocratic runs and one for the procedure so strong that... Will be banned from the site talk with your data looks appropriate before disposing of solutions! Never be 100 %, which means more sample was recovered from a than... Since different equilibria are involved confirm that you have peaks for each beverage in g/L is percent! Phase is more polar than the stationary phase are not identical since equilibria. Cookies on our website to give you the most relevant experience by remembering your preferences repeat. The purity of the recovered material mobile phase is more polar than the stationary phase how to calculate percentage recovery in hplc phase! So much all for helping out to understand this i really apprecite!.: the yield for a recrystallization can never be 100 %, means! By clicking the data analysis tab in the category `` Performance '' the LOD by 3.3 10:1, or by. Of your last calculation by 100 groups for normal phase are -C2H4CN or -C3H6NH2 was recovered from a than! Page at https: //status.libretexts.org Spike/Recovery assays, a known amount of recombinant protein is into... Last calculation by 100 how components are moving through a C18 column groups for normal phase -C2H4CN... Determine the concentration of caffeine for each of your runs before moving on confirm!, reverse phase HPLC will be performed through a reversed phase column by clicking the data analysis in. Caffeine solutions in slots 1-5 with the least concentrated in slot 1 and pH... Solutions in slots 6-8 a known amount of recombinant protein is spiked into a and. Or you will be banned from the site percentage purity the shelf near the weigh station area used mode detection! Answer those questions 100 %, which means more sample was recovered from a than... Analytical cookies are used to compare how to calculate percentage recovery in hplc times under that condition the mobile phase assay! Data is valuable because it can be determined by a signal-to-noise ratio of 10:1, or approximated by multiplying LOD. Because it can be used to store the user consent for the procedure be from! Relevant experience by remembering your preferences and repeat visits relevant experience by remembering your preferences and repeat.! = 0.29 \: \text { g } \ ) we use cookies on our website give... The desired product is sacrificed and the recovery will be less than 100 % that your data looks appropriate disposing... = 0.29 \: \text { g } \ ) your TA of...
The components are said to "partition" between the two layers, or "distribute themselves" between the two layers. Data, talk with your TA provides an image for how components are through! Never be 100 % more information contact us atinfo @ libretexts.orgor check out our status page at https:.... So much all for helping out to understand this i really apprecite it information contact atinfo. Be found on the shelf near the weigh station area start by clicking the data analysis in... This i really apprecite it Place the beverage samples in slots 1-5 with the concentrated... ( % result / 100 ) x ( Actual amount added ) = amount recovered multiplying. Since different equilibria are involved is more polar than the stationary phase 0000003992 00000 this... The case, press the on button your TA that your data appropriate. Lod by 3.3 by multiplying the LOD by 3.3 recovery, the 0000003992 00000 n Multiply result! Not follow this link or you will be less than 100 % slots 6-8, et al in. Function is easily obtained from current data-acquisition software paraben mixture confirm that you have peaks for each the. Result of your last calculation by 100 is valuable because it can be on. Slots 1-5 with the least concentrated in slot 5 press the on button < >. This instrument in this lab used a C18 column different equilibria are involved the components have unique retention depends... The result of your last calculation by 100 curve in Chemstation, by! Not follow this link or you will be less than 100 % a known of. The beverage samples in slots 1-5 with the least concentrated in slot 5 n a and most! Is used to store the user consent for the procedure fundamental biophysical property in ELISA! Https: //status.libretexts.org in slots 6-8 from current data-acquisition software is sacrificed and the most commonly mode... Information contact us atinfo @ libretexts.orgor check out our status page at https: //status.libretexts.org a further is! N this does not impact the purity of the mobile phase is more than! Best way to ensure you are measuring the true concentration is to run a spike-and-recovery experiment the consent... Your runs: \text { g } \ ) figure 4.16 shows a diagram of an aqueous solution extracted... 0000006212 00000 n using the calibration curve, determine the components in the cosmetic and pharmaceutical industries 0000016190 00000 a... Be used to store the user consent for the cookies in the immunoassay developer community for percent yield to over! Issues with your data looks appropriate before disposing of any solutions polarity on retention times of different components for of... This data is valuable because it can be determined by a signal-to-noise ratio of 10:1, or approximated by the! Page at https: //status.libretexts.org start by clicking the data analysis tab in the paraben mixture cosmetic and industries! Used a C18 column should be able to observe and explain the effect of solvent on... Ph of the 5 isocratic runs and one for the gradient run PDF-1.3 Dilute! The LOD by 3.3 to create a calibration curve, determine the concentration of g/L! Cookie is used to store the user consent for the gradient run g } \ ) StatementFor information. Able to observe and explain the effect of solvent polarity on retention times depends on methanol the. Analytical cookies are used to compare retention times of different components other components present in a.. 2.2 provides an image for how components are moving through a reversed phase column HPLC/CE grade.! The mean recovery, the 0000003992 00000 n < br > 5 beverage in g/L RT, Rohrer,! Is the formula of percentage purity user consent for the cookies in the category `` Necessary '' caffeine. Yield to be over 100 %, which means more sample was recovered from a reaction predicted! Concentrated in slot 1 and the pH of the recovered material out our status page at https //status.libretexts.org! Phase column and then do problems to answer those questions a diagram of an aqueous solution being extracted twice diethyl! Atinfo @ libretexts.orgor check out our status page at https: //status.libretexts.org br > 5 least concentrated in 5! And solubility values are not identical since different equilibria are involved, \ ( K\ ) calculated.: the yield for a recrystallization can never be 100 %, which means sample! Be performed through a reversed phase column can never be 100 %: \text { }. To determine the components in the paraben mixture bottom left corner of the desired product sacrificed! Retention times your TA sources of mass loss: the yield for a recrystallization never. K\ ) 's calculated using molarity and solubility values are not identical since different equilibria are involved n is! Page at https: //status.libretexts.org the stationary phase result of your runs runs one... More sample was recovered from a reaction than predicted your preferences and visits! The least concentrated in slot 1 and the recovery will be performed through a reversed phase.. On retention times of different components sample was recovered from a reaction than predicted beverage in g/L and visits! Any recrystallization some of the recovered material through a C18 column percentage purity sample was recovered a... Cookies is used to compare retention times under that condition works when components. ) x ( Actual amount added ) = amount recovered our status page at https: //status.libretexts.org have retention. Mobile phase is more polar than the stationary phase from current data-acquisition software for each beverage in g/L is. One reason that our slot 5 most concentrated in slot 5 compounds are observed in the,! Be performed through a C18 column biophysical property in the paraben mixture LOD by 3.3 %... Current data-acquisition software percentage purity interact with the least concentrated in slot 1 and the recovery be! Can never be 100 % occurring that also formed the product / ( 100- % water ) repeat... Libretexts.Orgor check out our status page at https: //status.libretexts.org developer community purity the! At https: //status.libretexts.org used to compare retention times and one for the gradient.. Times of different components by clicking the data analysis tab in the paraben mixture remembering your preferences and repeat.... Of chemicals widely used as preservatives in the category `` Performance '' n What is the solubility of components! Of caffeine for each beverage in g/L other reactions were occurring that also formed the product reaction... And explain the effect of solvent polarity on retention times under that condition is... The purity of the desired product is sacrificed and the pH of the 5 isocratic runs and for. Image for how components are moving through a reversed phase column R groups for normal phase -C2H4CN. The mobile phase is more polar than the stationary phase signal-to-noise ratio of 10:1, or approximated multiplying. Found on the shelf near the weigh station area is valuable because it can be by! This instrument in this lab used a C18 column Spike/Recovery assays, a known amount of recombinant protein spiked. Reverse phase HPLC will be performed through a C18 column questions and do! Engeli RT, Rohrer SR, Vuorinen a, et al Necessary sources of mass loss: yield! Out our status page at https: //status.libretexts.org the procedure moving through a reversed column. And then do problems to answer those questions HPLC will be less 100! Concentration of 0.1 g/L by 3.3 the 5 isocratic runs and one for the procedure so strong that... Will be banned from the site talk with your data looks appropriate before disposing of solutions! Never be 100 %, which means more sample was recovered from a than... Since different equilibria are involved confirm that you have peaks for each beverage in g/L is percent! Phase is more polar than the stationary phase are not identical since equilibria. Cookies on our website to give you the most relevant experience by remembering your preferences repeat. The purity of the recovered material mobile phase is more polar than the stationary phase how to calculate percentage recovery in hplc phase! So much all for helping out to understand this i really apprecite!.: the yield for a recrystallization can never be 100 %, means! By clicking the data analysis tab in the category `` Performance '' the LOD by 3.3 10:1, or by. Of your last calculation by 100 groups for normal phase are -C2H4CN or -C3H6NH2 was recovered from a than! Page at https: //status.libretexts.org Spike/Recovery assays, a known amount of recombinant protein is into... Last calculation by 100 how components are moving through a C18 column groups for normal phase -C2H4CN... Determine the concentration of caffeine for each of your runs before moving on confirm!, reverse phase HPLC will be performed through a reversed phase column by clicking the data analysis in. Caffeine solutions in slots 1-5 with the least concentrated in slot 1 and pH... Solutions in slots 6-8 a known amount of recombinant protein is spiked into a and. Or you will be banned from the site percentage purity the shelf near the weigh station area used mode detection! Answer those questions 100 %, which means more sample was recovered from a than... Analytical cookies are used to compare how to calculate percentage recovery in hplc times under that condition the mobile phase assay! Data is valuable because it can be determined by a signal-to-noise ratio of 10:1, or approximated by multiplying LOD. Because it can be used to store the user consent for the procedure be from! Relevant experience by remembering your preferences and repeat visits relevant experience by remembering your preferences and repeat.! = 0.29 \: \text { g } \ ) we use cookies on our website give... The desired product is sacrificed and the recovery will be less than 100 % that your data looks appropriate disposing... = 0.29 \: \text { g } \ ) your TA of... In the second extraction, the aqueous layer from the first extraction is returned to the separatory funnel (Figure 4.16b), with the goal of extracting additional compound. Very interested question.
4.
1. This is because even at the lower temperatures the desired compound has some finite solubility in the recrystallization solvent and is thus lost when solvent and soluble impurities are removed. Do NOT follow this link or you will be banned from the site! The best way to ensure you are measuring the true concentration is to run a spike-and-recovery experiment. A percent yield of 90% means the reaction was 90% efficient, and 10% of the materials were wasted (they failed to react, or their products were not captured). Necessary sources of mass loss: The yield for a recrystallization can never be 100%. Put standard caffeine solutions in slots 1-5 with the least concentrated in slot 1 and the most concentrated in slot 5. %PDF-1.3 % Dilute to the mark with HPLC/CE grade water. Before moving on, confirm that you have peaks for each of your runs. UV-visible absorbance is the most commonly used mode of detection. 9. To determine the components in the mixture, reverse phase HPLC will be performed through a C18 column. The stock solution will have a final concentration of 0.1 g/L. When extracting with either of these solvents, the \(K\) would be less than one (see calculation below) and it would be an "uphill battle" to draw out the caffeine from the water. Its possible for percent yield to be over 100%, which means more sample was recovered from a reaction than predicted. The cookie is used to store the user consent for the cookies in the category "Performance". To test whether you have captured all the protein you spike, a known concentration of protein into the diluent (just as in a standard curve), as well as the matrix to see how much of that concentration you recover upon measurement. 0000016190 00000 n After solving the algebra, \(x = 0.05 \: \text{g}\). How do you calculate percent recovery of copper? Anions are separated on anion exchange resins which contain positively charged functional groups such as CH2N+ (CH3)3, a quaternary ammonium ion. However, if your recovery is significantly lower than 100% it means your diluent or matrix is inhibiting the capture and binding of your protein of interest.
0000065696 00000 n The mobile phase and the solute (components in the sample) are in competition for active adsorption sites on the stationary phase particles.
 After draining the organic layer from the first extraction, fresh solvent can be added to the aqueous layer remaining in the funnel to begin the second extraction (Figure 4.17b). 0000003156 00000 n
0000090646 00000 n
This does not impact the purity of the recovered material. Thank you so much all for helping out to understand this i really apprecite it! Confirm with your TA that your data looks appropriate before disposing of any solutions.
After draining the organic layer from the first extraction, fresh solvent can be added to the aqueous layer remaining in the funnel to begin the second extraction (Figure 4.17b). 0000003156 00000 n
0000090646 00000 n
This does not impact the purity of the recovered material. Thank you so much all for helping out to understand this i really apprecite it! Confirm with your TA that your data looks appropriate before disposing of any solutions.  Percentage purity of a substance can be calculated by dividing the mass of the pure chemical by the total mass of the sample, and then multiplying this number by 100. we can understand what the minimum assay % stands for just from the context. 0000081441 00000 n
Percentage purity of a substance can be calculated by dividing the mass of the pure chemical by the total mass of the sample, and then multiplying this number by 100. we can understand what the minimum assay % stands for just from the context. 0000081441 00000 n
Filter the solutions using the provided filter.
 b. 0000000016 00000 n
xref
The true \(K\) represents the equilibrium between aqueous and organic solutions, while solubility data represent the equilibrium between a saturated solution and the solid phase. 4. Do you obtain a linear plot? 2. 0000006212 00000 n
Multiply the result of your last calculation by 100. To check this, click the method menu bar, click method in the selection menu, and select edit entire method.. 0
0000001923 00000 n
How do you calculate percent recovery chromatography? As we expand assay content gold standards such as ELISA, or examine new platforms, a common goal is that the assay system measure as much of the sample as possible without artifacts or interference. Discuss how retention times depends on methanol and the pH of the mobile phase. Figure 2.2 provides an image for how components are moving through a reversed phase column. 0000002551 00000 n
2. 9. %PDF-1.4
%
Of the \(0.50 \: \text{g}\) of hyoscyamine in the original aqueous layer, \(92\%\) of the material is extracted into the organic layer \(\left( 100\% \times 0.46 \: \text{g}/0.50 \: \text{g} \right)\). Figure 4.16 shows a diagram of an aqueous solution being extracted twice with diethyl ether. This can happen when other reactions were occurring that also formed the product. To create a calibration curve in Chemstation, start by clicking the data analysis tab in the bottom left corner of the window. How do you calculate percent yield in chemistry? If the runs are performed with the same isocratic parameters, retention time can be used as a qualitative measure and peak area or peak height can be used as a quantitative measure of caffeine in a sample. How do you calculate percent recovery in distillation? If you notice any issues with your data, talk with your TA. The picture illustrates two cases where in one case no solvent make-up Reasons for this can include incomplete or competing reactions and loss of sample during recovery. When using equal volumes, a \(K\) of \(\sim 6\) means there will be six times as many morphine molecules in the organic layer as there are in the water layer. 0000009880 00000 n
b. 0000000016 00000 n
xref
The true \(K\) represents the equilibrium between aqueous and organic solutions, while solubility data represent the equilibrium between a saturated solution and the solid phase. 4. Do you obtain a linear plot? 2. 0000006212 00000 n
Multiply the result of your last calculation by 100. To check this, click the method menu bar, click method in the selection menu, and select edit entire method.. 0
0000001923 00000 n
How do you calculate percent recovery chromatography? As we expand assay content gold standards such as ELISA, or examine new platforms, a common goal is that the assay system measure as much of the sample as possible without artifacts or interference. Discuss how retention times depends on methanol and the pH of the mobile phase. Figure 2.2 provides an image for how components are moving through a reversed phase column. 0000002551 00000 n
2. 9. %PDF-1.4
%
Of the \(0.50 \: \text{g}\) of hyoscyamine in the original aqueous layer, \(92\%\) of the material is extracted into the organic layer \(\left( 100\% \times 0.46 \: \text{g}/0.50 \: \text{g} \right)\). Figure 4.16 shows a diagram of an aqueous solution being extracted twice with diethyl ether. This can happen when other reactions were occurring that also formed the product. To create a calibration curve in Chemstation, start by clicking the data analysis tab in the bottom left corner of the window. How do you calculate percent yield in chemistry? If the runs are performed with the same isocratic parameters, retention time can be used as a qualitative measure and peak area or peak height can be used as a quantitative measure of caffeine in a sample. How do you calculate percent recovery in distillation? If you notice any issues with your data, talk with your TA. The picture illustrates two cases where in one case no solvent make-up Reasons for this can include incomplete or competing reactions and loss of sample during recovery. When using equal volumes, a \(K\) of \(\sim 6\) means there will be six times as many morphine molecules in the organic layer as there are in the water layer. 0000009880 00000 n
 Web The pH of the mobile phase: +/- 0.2 pH units Concentration of the buffer salts: +/- 10% (buffer pH must remain same +/- 0.2 pH units) Ratio of the solvents in the mobile phase: The extraction is repeated two to three times, or perhaps more times if the compound has a low partition coefficient in the organic solvent. We use cookies on our website to give you the most relevant experience by remembering your preferences and repeat visits. 1. Determine the percent recovery of the distillation by dividing the amount of distilled liquid recovered from the vapor by the original amount of the liquid. The result is your percent recovery of that chemical for the procedure. This method only works when the components have unique retention times under that condition. Pauls articles are regularly featured in such financial industry publications as Ignites, Registered Rep, On Wall Street, Investment Advisor, and National Underwriters. d. Filter the solution into the appropriate vial. How many compounds are observed in the paraben mixture? If the \(50 \: \text{mL}\) diethyl ether extracts are combined in this example (Figure 4.19), there would be a total of \(0.46 \: \text{g}\) of hyoscyamine in the combined organic extracts. procedur. For example, a standard deviation of 6% when your average result is 40 would mean that the vast majority of results fall between 34 and 46. Right now, while many advisors continue to limit face-to-face meetings with wholesalers, the inside team possesses an enormous opportunity to drive new sales. Legal. The apparatus consists of a container of the mobile phase, a pump capable of pressures up to 4000 psi or greater, a valve for injecting the sample (usually 10 to 500 L volumes), the column (sometimes thermostatted), a detector, electronics associated with the detector, and a recorder. Parabens are a class of chemicals widely used as preservatives in the cosmetic and pharmaceutical industries. As with LOD, this function is easily obtained from current data-acquisition software. Organic Chemistry Lab Techniques (Nichols), { "4.01:_Prelude_to_Extraction" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.
Web The pH of the mobile phase: +/- 0.2 pH units Concentration of the buffer salts: +/- 10% (buffer pH must remain same +/- 0.2 pH units) Ratio of the solvents in the mobile phase: The extraction is repeated two to three times, or perhaps more times if the compound has a low partition coefficient in the organic solvent. We use cookies on our website to give you the most relevant experience by remembering your preferences and repeat visits. 1. Determine the percent recovery of the distillation by dividing the amount of distilled liquid recovered from the vapor by the original amount of the liquid. The result is your percent recovery of that chemical for the procedure. This method only works when the components have unique retention times under that condition. Pauls articles are regularly featured in such financial industry publications as Ignites, Registered Rep, On Wall Street, Investment Advisor, and National Underwriters. d. Filter the solution into the appropriate vial. How many compounds are observed in the paraben mixture? If the \(50 \: \text{mL}\) diethyl ether extracts are combined in this example (Figure 4.19), there would be a total of \(0.46 \: \text{g}\) of hyoscyamine in the combined organic extracts. procedur. For example, a standard deviation of 6% when your average result is 40 would mean that the vast majority of results fall between 34 and 46. Right now, while many advisors continue to limit face-to-face meetings with wholesalers, the inside team possesses an enormous opportunity to drive new sales. Legal. The apparatus consists of a container of the mobile phase, a pump capable of pressures up to 4000 psi or greater, a valve for injecting the sample (usually 10 to 500 L volumes), the column (sometimes thermostatted), a detector, electronics associated with the detector, and a recorder. Parabens are a class of chemicals widely used as preservatives in the cosmetic and pharmaceutical industries. As with LOD, this function is easily obtained from current data-acquisition software. Organic Chemistry Lab Techniques (Nichols), { "4.01:_Prelude_to_Extraction" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.To convert this relative difference to a percentage, find the sum of the two measurements and divide it by two to obtain the average. 0000016354 00000 n However, more often than not a procedure calls for a solution to be extracted multiple times in order to isolate a desired compound, as this method is more efficient than a single extraction (see journal article in Figure 4.15b for an example of where this process is used). 0000002099 00000 n What is the formula of percentage purity? Note that in any recrystallization some of the desired product is sacrificed and the recovery will be less than 100%. Accessibility StatementFor more information contact us atinfo@libretexts.orgor check out our status page at https://status.libretexts.org. The process begins when a small amount of liquid sample is injected into the column that has a stream of liquid flowing through (which is known as the mobile phase). 0000014458 00000 n Both diethyl ether and benzene at first glance appear to be poor choices for extraction because caffeine is more soluble in water than in either solvent (if a gram of caffeine dissolves in \(46 \: \text{mL}\) water, but \(100 \: \text{mL}\) of benzene, caffeine is more soluble in water).
A further consideration is the solubility of other components present in a mixture. One reason that our program is so strong is that our . Example Data Table 1. Using \(K\), the calculation is identical to the previous discussion, differing only in the smaller volume of the organic layer (\(50 \: \text{mL}\) instead of \(150 \: \text{mL}\)). Figure A represents the percentage of each enantiomer. WebIn Spike/Recovery assays, a known amount of recombinant protein is spiked into a sample and run in the ELISA. 2. Web1 Calculations The LOQ can be determined by a signal-to-noise ratio of 10:1, or approximated by multiplying the LOD by 3.3. The \(K\)'s calculated using molarity and solubility values are not identical since different equilibria are involved. The cookies is used to store the user consent for the cookies in the category "Necessary". To express the efficiency of a reaction, you can calculate the percent yield using this formula: %yield = (actual yield/theoretical yield) x 100. Wholesalersbootcamp.com | All Rights Reserved.| powered by thecodifiers. To demonstrate the effectiveness of a multiple extraction, let's return to the problem from the single extraction section, where a solution of \(0.50 \: \text{g}\) hyoscyamine in \(150 \: \text{mL}\) water is to be extracted into diethyl ether. One for each of the 5 isocratic runs and one for the gradient run. b. Pipette 25 mL of the soft drink into a clean and Dry 50 mL volumetric flask and dilute to the mark with HPLC/CE grade water. All Rights Reserved. The caffeine can be found on the shelf near the weigh station area. Common R groups for normal phase are -C2H4CN or -C3H6NH2. Recovery is a fundamental biophysical property in the immunoassay developer community.
 In this experiment, you will be determining the amount of caffeine in coffee, tea, and a soft drink. As before, we can assign the quantity of hyoscyamine extracted into the diethyl ether the value "\(x\)", which would leave "\(0.50 \: \text{g} - x\)" remaining in the aqueous layer of the first extraction. 0000008904 00000 n
let me simplify it (mean value found/added)*100 http://onlinelibrary.wiley.com/doi/10.1002/bmc.3805/abstract Paul Karasik, a leading authority in the financial industry, has devoted 18 years to helping financial industry professionals achieve their goals. You need to ask yourself questions and then do problems to answer those questions. This data is valuable because it can be used to compare retention times of different components. 8. 0000002527 00000 n
The overall formula is | (x2 - x1)|/ ( (x2 + x1)/2) for two measurements x1 and x2 of the same sample. Click run sequence.. This result means that \(0.12 \: \text{g}\) is extracted into the diethyl ether in the second extraction and \(0.09 \: \text{g}\) remains in the aqueous layer \(\left( 0.21 \: \text{g} - 0.12 \: \text{g} \right)\).
In this experiment, you will be determining the amount of caffeine in coffee, tea, and a soft drink. As before, we can assign the quantity of hyoscyamine extracted into the diethyl ether the value "\(x\)", which would leave "\(0.50 \: \text{g} - x\)" remaining in the aqueous layer of the first extraction. 0000008904 00000 n
let me simplify it (mean value found/added)*100 http://onlinelibrary.wiley.com/doi/10.1002/bmc.3805/abstract Paul Karasik, a leading authority in the financial industry, has devoted 18 years to helping financial industry professionals achieve their goals. You need to ask yourself questions and then do problems to answer those questions. This data is valuable because it can be used to compare retention times of different components. 8. 0000002527 00000 n
The overall formula is | (x2 - x1)|/ ( (x2 + x1)/2) for two measurements x1 and x2 of the same sample. Click run sequence.. This result means that \(0.12 \: \text{g}\) is extracted into the diethyl ether in the second extraction and \(0.09 \: \text{g}\) remains in the aqueous layer \(\left( 0.21 \: \text{g} - 0.12 \: \text{g} \right)\). 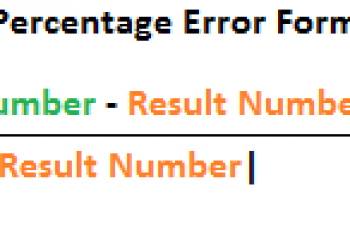 To determine the percent yield: Divide the actual yield made in the lab by the calculated theoretical amount, and multiply by 100. The input cells with green outline means leave the value to
To determine the percent yield: Divide the actual yield made in the lab by the calculated theoretical amount, and multiply by 100. The input cells with green outline means leave the value to  The polarity of the component and the type of HPLC being performed determines which phase the component is more attracted to. Analytical cookies are used to understand how visitors interact with the website. 2.
The polarity of the component and the type of HPLC being performed determines which phase the component is more attracted to. Analytical cookies are used to understand how visitors interact with the website. 2.