Note that HPO terms are normalized as detailed in Alag 2020 [6]. Subgroups After primary analysis, often want to look at subgroups Does effectiveness vary by subgroup If drug effective, is it more effective in some populations? Further, the publicly-available site (http://CovidResearchTrials.com) contains analysis at multiple time points, further providing researchers with longitudinal information about clinical trials and associated entities, as well as demonstrating the reproducibility of the methods. Eligibility criteria When deciding on these criteria, using excessive restrictions in all effort to obtain a pure (or homogenous) sample can lead to extreme difficulty in getting sufficient participants. Since the onset of the Coronavirus disease 2019 (COVID-19) pandemic, researchers and clinicians have been swiftly conducting clinical trials to better understand the virus, its transmission, and potential drugs and vaccines to counter its rapid spread. what are clinical trials?. The term clinical trial is preferred over clinical experiment because the latter may connote disrespect for the value of human life. If an intervention is shown to be successful or unsuccessful, the medical and scientific communities must know what kinds of people the finding apply. 

 The primary response variable must be capable of being assessed in all participants. Web2 Assessments A combination of coursework and practical training in clinical trials documentation and standards provides the skills necessary to prepare you for a career as a SAS Certified Clinical Trials Programming Professional. carl h. coleman professor of law director, health law, Predictive Analysis of Clinical Trials - . overview, Clinical Trials - . Why are clinical trials needed? As of late August 2020 there were nearly 3,500 COVID-19 related clinical trials. It is important to have response variable that can be ascertained as completely as possible. Insights from analyzing COVID-19 clinical trials. gcrc, vanderbilt university medical center. WebA necessary companion to well-designed clinical trial is its appropriate statistical analysis. Fig 3 depicts the frequency of intervention types across COVID-19 related trials, illustrating that the most popular intervention category is Drug, followed by Other, Behavioral, Biological, and Diagnostic test. Types of trials Therapeutic trials measure the efficacy of drugs or other therapeutic procedures (for example: diet, bed-rest, surgery, physiotherapy and ionizing radiation). Design is the process or structure that isolates the factors of interest.
The primary response variable must be capable of being assessed in all participants. Web2 Assessments A combination of coursework and practical training in clinical trials documentation and standards provides the skills necessary to prepare you for a career as a SAS Certified Clinical Trials Programming Professional. carl h. coleman professor of law director, health law, Predictive Analysis of Clinical Trials - . overview, Clinical Trials - . Why are clinical trials needed? As of late August 2020 there were nearly 3,500 COVID-19 related clinical trials. It is important to have response variable that can be ascertained as completely as possible. Insights from analyzing COVID-19 clinical trials. gcrc, vanderbilt university medical center. WebA necessary companion to well-designed clinical trial is its appropriate statistical analysis. Fig 3 depicts the frequency of intervention types across COVID-19 related trials, illustrating that the most popular intervention category is Drug, followed by Other, Behavioral, Biological, and Diagnostic test. Types of trials Therapeutic trials measure the efficacy of drugs or other therapeutic procedures (for example: diet, bed-rest, surgery, physiotherapy and ionizing radiation). Design is the process or structure that isolates the factors of interest.  Need for randomization Three possibilities of why the observed difference between the two groups is not due to chance: The two groups differ appreciably in factors related to their prognosis. WebClinical Trials Market | Industry Analysis Report, 2018-2025 - A new market study based on the Clinical Trials Market designed from various sources which also include porter's five forces analysis research techniques to explore the new opening of the market for the period of 2019-2025. Response variable should be capable of unbiased assessment. Electronic Clinical Trials - Electronic clinical trials have come into vogue in the clinical trials management system. Patients receiving A are two to six years old and parents receiving B are either younger or older. For more information about PLOS Subject Areas, click Problems in timing of clinical trials Trials need to be feasible: feasibility includes knowledge, tools, knowledge on safety of intervention, outcomes to be assessed. 2. A clinical trial actually is an experiment testing medical treatments on human subjects. (Piantadosi 2005), To establish a hypothesis requires both a theoretical basis in biology and statistical support for the hypothesis, based on the observed data and the theoretical statistical model. chief, biometric research branch national cancer. International Clinical Trials - . Gulliford, M. C., O. C. Ukoumunne, et al. paolo bruzzi clinical epidemiology unit national cancer, Ethical Issues in Clinical Trials in Developing Countries - .
Need for randomization Three possibilities of why the observed difference between the two groups is not due to chance: The two groups differ appreciably in factors related to their prognosis. WebClinical Trials Market | Industry Analysis Report, 2018-2025 - A new market study based on the Clinical Trials Market designed from various sources which also include porter's five forces analysis research techniques to explore the new opening of the market for the period of 2019-2025. Response variable should be capable of unbiased assessment. Electronic Clinical Trials - Electronic clinical trials have come into vogue in the clinical trials management system. Patients receiving A are two to six years old and parents receiving B are either younger or older. For more information about PLOS Subject Areas, click Problems in timing of clinical trials Trials need to be feasible: feasibility includes knowledge, tools, knowledge on safety of intervention, outcomes to be assessed. 2. A clinical trial actually is an experiment testing medical treatments on human subjects. (Piantadosi 2005), To establish a hypothesis requires both a theoretical basis in biology and statistical support for the hypothesis, based on the observed data and the theoretical statistical model. chief, biometric research branch national cancer. International Clinical Trials - . Gulliford, M. C., O. C. Ukoumunne, et al. paolo bruzzi clinical epidemiology unit national cancer, Ethical Issues in Clinical Trials in Developing Countries - .  No, Is the Subject Area "Respiratory physiology" applicable to this article? Differential exclusion or withdrawal of subjects from the study Example: elimination of patients who were judged ineligible after enrollment by personnel who were aware of treatment assignment and course of treatment. https://doi.org/10.1371/journal.pone.0239694.g003. Cluster randomization: JAMA study 46 clinicians 401 patients, Cluster randomization of Clinicians ~10 patients ~10 patients ~10 patients ~10 patients ~10 patients ~10 patients 46 clinicians, 401 patients ~10 patients ~10 patients 100 kids, Cluster randomization of Clinicians ~10 patients ~10 patients ~10 patients ~10 patients 100 kids ~10 patients ~10 patients ~10 patients ~10 patients 46 clinicians, 401 patients, Subgroups Recommendations in NEJM(Wang et al) 1/25/11, Cluster randomization: Analysis Analysis must account for randomization of clusters, not individuals Most commonly used technique: Generalized Estimating Equations (GEE) Type of multiple regression In Stata and SAS Effective sample size is between total n and number of clusters, Cluster randomization: Sample size 1. joseph f. merola, md mmsc director of clinical trials, bwh dermatology 1/9/2014, Clinical Trials- - Clinical trial is performed when medicine or new medications are being tried for public use, during a, Clinical Trials - . N Engl J Med 2007; 357; 21: 2189-2194. Analysis of trials with continuous outcomes:Badly behaved outcomes Use t-test usually If radically non-normal, use non-parametric analogue Examples 1. cigarettes per day 2. From Lagakos SW, (2006). Web More than 8 years of experience working in the field of Clinical Data Management delivering Data Review and Management objectives in various clinical studies and phases in a timely manner. february 10, 2010. case 1. ppis in gi bleeding.
No, Is the Subject Area "Respiratory physiology" applicable to this article? Differential exclusion or withdrawal of subjects from the study Example: elimination of patients who were judged ineligible after enrollment by personnel who were aware of treatment assignment and course of treatment. https://doi.org/10.1371/journal.pone.0239694.g003. Cluster randomization: JAMA study 46 clinicians 401 patients, Cluster randomization of Clinicians ~10 patients ~10 patients ~10 patients ~10 patients ~10 patients ~10 patients 46 clinicians, 401 patients ~10 patients ~10 patients 100 kids, Cluster randomization of Clinicians ~10 patients ~10 patients ~10 patients ~10 patients 100 kids ~10 patients ~10 patients ~10 patients ~10 patients 46 clinicians, 401 patients, Subgroups Recommendations in NEJM(Wang et al) 1/25/11, Cluster randomization: Analysis Analysis must account for randomization of clusters, not individuals Most commonly used technique: Generalized Estimating Equations (GEE) Type of multiple regression In Stata and SAS Effective sample size is between total n and number of clusters, Cluster randomization: Sample size 1. joseph f. merola, md mmsc director of clinical trials, bwh dermatology 1/9/2014, Clinical Trials- - Clinical trial is performed when medicine or new medications are being tried for public use, during a, Clinical Trials - . N Engl J Med 2007; 357; 21: 2189-2194. Analysis of trials with continuous outcomes:Badly behaved outcomes Use t-test usually If radically non-normal, use non-parametric analogue Examples 1. cigarettes per day 2. From Lagakos SW, (2006). Web More than 8 years of experience working in the field of Clinical Data Management delivering Data Review and Management objectives in various clinical studies and phases in a timely manner. february 10, 2010. case 1. ppis in gi bleeding.  https://doi.org/10.1371/journal.pone.0239694.g005.
https://doi.org/10.1371/journal.pone.0239694.g005.  Statistics in Medicine - Reporting of Subgroup, Analyses in Clinical Trials. This case study template highlights the three important phases: patient data research, design clinical trials, and post evaluation statistics. Writing review & editing, Affiliation Multiple comparisons The general problem Each statistical test has a 5% chance of Type I error We are wrong 1 time out of 20 Easy to come up with spurious results Take a worthless drug (placebo 2) compare to placebo 1 1 study: P(type I error)= 5% 2 studies: P(1 or 2 type I errors)= almost 10% 20 studies: P(at least one significant)=64% Publication bias (Huge problem in genomic studies), Multiple comparisons: solutions? Lab and other special units c. Clinical center(s) 2. The number of COVID-19 related clinical trials is dramatically increasing: There were approximately 500 clinical trials in mid-late April, more than 1000 in early May, over 2000 in early June, and over 3000 in mid-July [1]. Additionally, the additional feature of correlating COVID-19 clinical trials to SNPs and protein mutations is provided through utilizing the HPO ontology and previous work in Alag 2020 [6]. ClinicalTrials.gov staff developed the online presentations listed below to help sponsors and investigators register studies on and submit results to ClinicalTrials.gov. PDF handouts and transcripts of each presentation are provided. The running time for each presentation is given in parentheses (minutes:seconds) after the presentation's title. Withdrawal studies Studies in which the participant on a particular treatment for a chronic disease are taken off therapy or have the dosage reduced. Except where otherwise noted, content on this site is licensed under a CC BY-NC 4.0 license. Relative stability of intervention: If the intended intervention becomes outmoded in short duration, studying such intervention may be inappropriate. Adverse effect Most interventions are likely to have adverse effects. It will cover the study protocol, eCRFs (capture of data) WebIn clinical trials and other scientific studies, an interim analysis is an analysis of data that is conducted before data collection has been completed. Worked on multiple projects simultaneously with taking little or no guidance from the project managers. In order for other investigators to be able to replicate the study, they need data descriptive of the participants enrolled. Example: Suppose treatments A and B are assigned to two groups of children with leukemia. voluptates consectetur nulla eveniet iure vitae quibusdam?
Statistics in Medicine - Reporting of Subgroup, Analyses in Clinical Trials. This case study template highlights the three important phases: patient data research, design clinical trials, and post evaluation statistics. Writing review & editing, Affiliation Multiple comparisons The general problem Each statistical test has a 5% chance of Type I error We are wrong 1 time out of 20 Easy to come up with spurious results Take a worthless drug (placebo 2) compare to placebo 1 1 study: P(type I error)= 5% 2 studies: P(1 or 2 type I errors)= almost 10% 20 studies: P(at least one significant)=64% Publication bias (Huge problem in genomic studies), Multiple comparisons: solutions? Lab and other special units c. Clinical center(s) 2. The number of COVID-19 related clinical trials is dramatically increasing: There were approximately 500 clinical trials in mid-late April, more than 1000 in early May, over 2000 in early June, and over 3000 in mid-July [1]. Additionally, the additional feature of correlating COVID-19 clinical trials to SNPs and protein mutations is provided through utilizing the HPO ontology and previous work in Alag 2020 [6]. ClinicalTrials.gov staff developed the online presentations listed below to help sponsors and investigators register studies on and submit results to ClinicalTrials.gov. PDF handouts and transcripts of each presentation are provided. The running time for each presentation is given in parentheses (minutes:seconds) after the presentation's title. Withdrawal studies Studies in which the participant on a particular treatment for a chronic disease are taken off therapy or have the dosage reduced. Except where otherwise noted, content on this site is licensed under a CC BY-NC 4.0 license. Relative stability of intervention: If the intended intervention becomes outmoded in short duration, studying such intervention may be inappropriate. Adverse effect Most interventions are likely to have adverse effects. It will cover the study protocol, eCRFs (capture of data) WebIn clinical trials and other scientific studies, an interim analysis is an analysis of data that is conducted before data collection has been completed. Worked on multiple projects simultaneously with taking little or no guidance from the project managers. In order for other investigators to be able to replicate the study, they need data descriptive of the participants enrolled. Example: Suppose treatments A and B are assigned to two groups of children with leukemia. voluptates consectetur nulla eveniet iure vitae quibusdam?  are compared to patients who do not receive that intervention. Unless there is a combination of primary response variable in which the participant remains at risk of having additional events, participation generally ends when primary response variable occurs. Data analysis Interim analysis Final analysis 8. Ans: Guinness, When is a T-test Valid? Those implied by study protocol eg. For more information about PLOS Subject Areas, click bernard lo, m.d. Webdocumented before any exploratory data analysis, while post-hoc is not. richard simon, d.sc. May be framed in the form of testing of hypothesis. Factorial Designs Attempts to evaluate two interventions with a control in a single experiment Incomplete factorial designs when it is inappropriate, infeasible or unethical to address every possible treatment combination, Merits of factorial design A very essential design when there are two or more interventions Allow effects of one intervention to be estimated at all the levels of the other intervention Demerits of factorial design The basic concern in a factorial design is the existence of possible interaction and its impact on the sample size When there are two separate outcomes, (eg: heart disease and cancer) but one of the interventions have effect on both, then data monitoring becomes complicated or sometimes impossible, 2023 SlideServe | Powered By DigitalOfficePro, - - - - - - - - - - - - - - - - - - - - - - - - - - - E N D - - - - - - - - - - - - - - - - - - - - - - - - - - -.
are compared to patients who do not receive that intervention. Unless there is a combination of primary response variable in which the participant remains at risk of having additional events, participation generally ends when primary response variable occurs. Data analysis Interim analysis Final analysis 8. Ans: Guinness, When is a T-test Valid? Those implied by study protocol eg. For more information about PLOS Subject Areas, click bernard lo, m.d. Webdocumented before any exploratory data analysis, while post-hoc is not. richard simon, d.sc. May be framed in the form of testing of hypothesis. Factorial Designs Attempts to evaluate two interventions with a control in a single experiment Incomplete factorial designs when it is inappropriate, infeasible or unethical to address every possible treatment combination, Merits of factorial design A very essential design when there are two or more interventions Allow effects of one intervention to be estimated at all the levels of the other intervention Demerits of factorial design The basic concern in a factorial design is the existence of possible interaction and its impact on the sample size When there are two separate outcomes, (eg: heart disease and cancer) but one of the interventions have effect on both, then data monitoring becomes complicated or sometimes impossible, 2023 SlideServe | Powered By DigitalOfficePro, - - - - - - - - - - - - - - - - - - - - - - - - - - - E N D - - - - - - - - - - - - - - - - - - - - - - - - - - -.  Avoids between participant variation in estimating the intervention effect. trials to show superiority trials to. The freely available, novel resources present up-to-date relevant biological information and insights in a robust, accessible manner, illustrating their invaluable potential to aid researchers overcome COVID-19 and save hundreds of thousands of lives. Objective: Assess response to the discontinuation or reduction. By doing so, there are. The primary question as well as any secondary or subsidiary question, should be carefully selected, clearly defined and stated in advance. bernard lo, m.d. Although subject to change, the Covid Research Trials home page provides the latest analysis results. robert m califf md vice chancellor for clinical research. https://doi.org/10.1371/journal.pone.0239694.g002. For comparative trials, the window usually exists relatively early in the development of a new therapy. The Classical Approach. Data Analysis Issues in Clinical Trials.
Avoids between participant variation in estimating the intervention effect. trials to show superiority trials to. The freely available, novel resources present up-to-date relevant biological information and insights in a robust, accessible manner, illustrating their invaluable potential to aid researchers overcome COVID-19 and save hundreds of thousands of lives. Objective: Assess response to the discontinuation or reduction. By doing so, there are. The primary question as well as any secondary or subsidiary question, should be carefully selected, clearly defined and stated in advance. bernard lo, m.d. Although subject to change, the Covid Research Trials home page provides the latest analysis results. robert m califf md vice chancellor for clinical research. https://doi.org/10.1371/journal.pone.0239694.g002. For comparative trials, the window usually exists relatively early in the development of a new therapy. The Classical Approach. Data Analysis Issues in Clinical Trials. 
![]() In essence, for a given term of interest compute other correlated terms [.
In essence, for a given term of interest compute other correlated terms [.
Smeeth, L. and E. S. Ng (2002). Truly, double blind studies have a distinct advantage over other studies in this regard. In selecting participants to be studied, not only does the investigator requires people in whom the intervention might be worth, he also wants to choose people In whom there is a high likelihood that he can detect the hypothesized results of the intervention. This methodology and the generated reports provide a succinct summary of COVID-19 related Interventions/Drugs, MeSH terms, HPO terms, clinical trials, genes, SNPs, and protein mutations all in one place.
Formal analysis, As the field of statistics, the theoretical science or formal study of the inferential process, especially the planning and analysis of experiments, surveys, and observational studies. (Piantadosi 2005).  This data can be categorical or continuous.
This data can be categorical or continuous.  WebFor each clinical trial a risk assessment should generally be undertaken at the protocol development stage. none. short course presenters: michael krams, m.d., wyeth research vladimir dragalin, ph.d., wyeth. As a result of the unprecedented volume of new clinical trials, the task of staying informed about crucial developments and ongoing research is not only arduous but also extremely time-consuming. The Results section is comprised of discussions about the following three main areas: All analysis results are accessible via the Covid Research Trials home page, available at http://CovidResearchTrials.com. [brief] update. Readers are encouraged to refer to Alag 2020 for more in-depth details. Randomization is a problem for physicians to randomly assign a therapy if the investigator believes that a preferred therapy exists. WebClinical trials are designed with a number of parameters to generate accurate results. Generate unique publicly-accessible, informativeyet concisereports for each of the Intervention/Drug terms, MeSH terms, and HPO terms. CLINICAL TRIALS - . An independent assessment of 5 clinical trials in 2012 found that 89% of expected professional charges related to participant study visits were potentially unbilled. Funding: The author received no specific funding for this work. (In such case the best approach is to postpone the trial until a procedure has reached a plateau and is unlikely to change). Analyze the clinical trials at multiple time points, enabling future meta-analyses.
WebFor each clinical trial a risk assessment should generally be undertaken at the protocol development stage. none. short course presenters: michael krams, m.d., wyeth research vladimir dragalin, ph.d., wyeth. As a result of the unprecedented volume of new clinical trials, the task of staying informed about crucial developments and ongoing research is not only arduous but also extremely time-consuming. The Results section is comprised of discussions about the following three main areas: All analysis results are accessible via the Covid Research Trials home page, available at http://CovidResearchTrials.com. [brief] update. Readers are encouraged to refer to Alag 2020 for more in-depth details. Randomization is a problem for physicians to randomly assign a therapy if the investigator believes that a preferred therapy exists. WebClinical trials are designed with a number of parameters to generate accurate results. Generate unique publicly-accessible, informativeyet concisereports for each of the Intervention/Drug terms, MeSH terms, and HPO terms. CLINICAL TRIALS - . An independent assessment of 5 clinical trials in 2012 found that 89% of expected professional charges related to participant study visits were potentially unbilled. Funding: The author received no specific funding for this work. (In such case the best approach is to postpone the trial until a procedure has reached a plateau and is unlikely to change). Analyze the clinical trials at multiple time points, enabling future meta-analyses.
A brewery Quiz 1: Which brewery did Student work for? Inclusion criteria b. Exclusion criteria 2. Overview of simple data analysis for clinical trials Data analysis for non-standard study designs Cross over Cluster Assessment of eligibility c. Baseline examination d. Intervention allocation (e.g. type of comparisons. Similar requirements exist for the marketing of vaccines. WebSAS/Statistical programmer with over 7+ years of experience in clinical data analysis and working for Contract Research Organization(CRO), pharmaceutical and Biotechnology industries.Extensive expertise in handling and processing programs using SAS BASE, SAS/STAT, SAS/MACROS, SAS /ACCESS, etc.Excellent in variousSASprocedures for "Components of variance and intraclass correlations for the design of community-based surveys and intervention studies: data from the Health Survey for England 1994." 3. If results overall show no effect, does drug work in subgroup of participants? Webdata analysis in clinical trials ppt. In both medical and statistical sciences, empirical knowledge is generated from observations and data. However, any participant for whom the intervention is known to be harmful should not be admitted to the trial. Ethical issues in clinical trials - . No, Is the Subject Area "Pneumonia" applicable to this article? At baseline, the control group must be similar in relevant respects to the intervention group so that differences in the outcome may reasonably be attributed to action of intervention. Interventions can be drugs, medical devices, vaccines, procedures, genetic tests, noninvasive techniques, such as diet, education, or exercise, and are sorted into eleven different categories (e.g., genetic, radiation, etc.). research studies involving, Clinical trials - . The talk will be a microcosm of a clinical trial study. Therefore, improved accessibility of information about COVID-19 related clinical trials would aid clinicians, researchers, and the lay public alike. Further, all reports contain the details of the clinical trials in which the term is referenced.  A short history of the evolution of standards in clinical trials will be provided. Friedman, Furer and Demets.
A short history of the evolution of standards in clinical trials will be provided. Friedman, Furer and Demets. 
 richard simon, d.sc. Additionally, the home page has links to numerous Java APIs and a Google Colab page, which facilitate easy local access to this researchs insights and results. (1999). Software, Details on the created public repository to provide access to the data used, reports created, correlations mapped, and APIs produced. Choose the study design, and define the study population, predictor variables, and outcome variables ; measure these variables and anticipate problems with
richard simon, d.sc. Additionally, the home page has links to numerous Java APIs and a Google Colab page, which facilitate easy local access to this researchs insights and results. (1999). Software, Details on the created public repository to provide access to the data used, reports created, correlations mapped, and APIs produced. Choose the study design, and define the study population, predictor variables, and outcome variables ; measure these variables and anticipate problems with 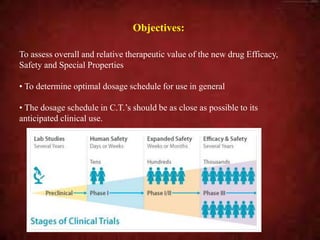
Participating investigators a. These parameter standards include a series of studies and evaluation. WebDeveloped SAS programs and macros to import, clean and analyze data and generated complex listings, graphs and reports for clinical team review. These terms were selected after reviewing various sites, including clinicaltrials.gov [10], that have kept a running list of COVID-19-related publications and clinical trials. Study population Definition The study population is the subset of the population with the condition or characteristic of interest defined by the eligibility criteria. c) How each patients response is to be assessed? Response variable Combining events to make up a response variable might be useful if any one event occurs infrequently for the investigator reasonably to expect a significant difference without using a large number of participants. Non accural: in which the subjects needed are recruited before the study begins.  Data Availability: All of the data used in this research is publically available at http://ClinicalTrials.gov.
Data Availability: All of the data used in this research is publically available at http://ClinicalTrials.gov.
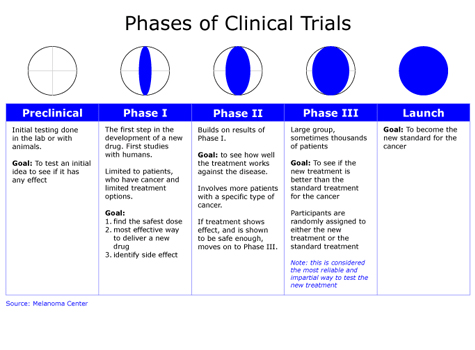 This guidance is intended to give direction to sponsors in the design, conduct, analysis, and evaluation of clinical trials of an investigational product in the context of its overall clinical development. The core of the pipeline and methodology is publicly available at protocols.io (dx.doi.org/10.17504/protocols.io.bfacjiaw). As detailed in the protocols, the following online repositories/vocabularies were used: The predominant differences between the methodology employed here and that followed in Alag 2020 [6] are detailed in the following subsections. A package titled cgmanalysis was developed in the free programming language R to provide a rapid, easy, and consistent methodology for CGM data management, summary measure calculation, and descriptive analysis. Survival, duration of response, and time until event data - Creative Commons Attribution NonCommercial License 4.0.
This guidance is intended to give direction to sponsors in the design, conduct, analysis, and evaluation of clinical trials of an investigational product in the context of its overall clinical development. The core of the pipeline and methodology is publicly available at protocols.io (dx.doi.org/10.17504/protocols.io.bfacjiaw). As detailed in the protocols, the following online repositories/vocabularies were used: The predominant differences between the methodology employed here and that followed in Alag 2020 [6] are detailed in the following subsections. A package titled cgmanalysis was developed in the free programming language R to provide a rapid, easy, and consistent methodology for CGM data management, summary measure calculation, and descriptive analysis. Survival, duration of response, and time until event data - Creative Commons Attribution NonCommercial License 4.0.